Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells
- PMID: 22566825
- PMCID: PMC3342056
- DOI: 10.3389/fimmu.2011.00035
Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells
Abstract
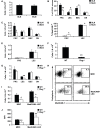
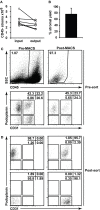
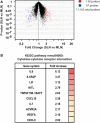
Within lymph nodes, non-hematopoietic stromal cells organize and interact with leukocytes in an immunologically important manner. In addition to organizing T and B cell segregation and expressing lymphocyte survival factors, several recent studies have shown that lymph node stromal cells shape the naïve T cell repertoire, expressing self-antigens which delete self-reactive T cells in a unique and non-redundant fashion. A fundamental role in peripheral tolerance, in addition to an otherwise extensive functional portfolio, necessitates closer study of lymph node stromal cell subsets using modern immunological techniques; however this has not routinely been possible in the field, due to difficulties reproducibly isolating these rare subsets. Techniques were therefore developed for successful ex vivo and in vitro manipulation and characterization of lymph node stroma. Here we discuss and validate these techniques in mice and humans, and apply them to address several unanswered questions regarding lymph node composition. We explored the steady-state stromal composition of lymph nodes isolated from mice and humans, and found that marginal reticular cells and lymphatic endothelial cells required lymphocytes for their normal maturation in mice. We also report alterations in the proportion and number of fibroblastic reticular cells (FRCs) between skin-draining and mesenteric lymph nodes. Similarly, transcriptional profiling of FRCs revealed changes in cytokine production from these sites. Together, these methods permit highly reproducible stromal cell isolation, sorting, and culture.
Keywords: endothelial cells; fibroblastic reticular cells; human lymph nodes; lymph node stromal cells; mouse lymph nodes; stromal cells; tolerance.
Figures



References
-
- Ahrendt M., Hammerschmidt S. I., Pabst O., Pabst R., Bode U. (2008). Stromal cells confer lymph node-specific properties by shaping a unique microenvironment influencing local immune responses. J. Immunol. 181, 1898–1907 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

