Mast cells as cellular sensors in inflammation and immunity
- PMID: 22566827
- PMCID: PMC3342044
- DOI: 10.3389/fimmu.2011.00037
Mast cells as cellular sensors in inflammation and immunity
Abstract
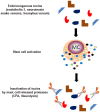
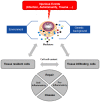
Mast cells are localized in tissues. Intense research on these cells over the years has demonstrated their role as effector cells in the maintenance of tissue integrity following injury produced by infectious agents, toxins, metabolic states, etc. After stimulation they release a sophisticated array of inflammatory mediators, cytokines, and growth factors to orchestrate an inflammatory response. These mediators can directly initiate tissue responses on resident cells, but they have also been shown to regulate other infiltrating immune cell functions. Research in recent years has revealed that the outcome of mast cell actions is not always detrimental for the host but can also limit disease development. In addition, mast cell functions highly depend on the physiological context in the organism. Depending on the genetic background, strength of the injurious event, the particular microenvironment, mast cells direct responses ranging from pro- to anti-inflammatory. It appears that they have evolved as cellular sensors to discern their environment in order to initiate an appropriate physiological response either aimed to favor inflammation for repair or at the contrary limit the inflammatory process to prevent further damage. Like every sophisticated machinery, its dysregulation leads to pathology. Given the broad distribution of mast cells in tissues this also explains their implication in many inflammatory diseases.
Keywords: immunity; inflammation; mast cells.
Figures
References
-
- Abonia J. P., Friend D. S., Austen W. G., Jr., Moore F. D., Jr., Carroll M. C., Chan R., Afnan J., Humbles A., Gerard C., Knight P., Kanaoka Y., Yasuda S., Morokawa N., Austen K. F., Stevens R. L., Gurish M. F. (2005). Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J. Immunol. 174, 7285–7291 - PMC - PubMed
-
- Ahmad A., Wang C. H., Bell R. G. (1991). A role for IgE in intestinal immunity. Expression of rapid expulsion of Trichinella spiralis in rats transfused with IgE and thoracic duct lymphocytes. J. Immunol. 146, 3563–3570 - PubMed
LinkOut - more resources
Full Text Sources

