Modulation of the inhibitory receptor leukocyte Ig-like receptor 1 on human natural killer cells
- PMID: 22566836
- PMCID: PMC3342057
- DOI: 10.3389/fimmu.2011.00046
Modulation of the inhibitory receptor leukocyte Ig-like receptor 1 on human natural killer cells
Abstract
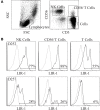
Leukocyte Ig-like receptor 1 (LIR-1) is an inhibitory Ig superfamily receptor with broad specificity for MHC-I expressed on leukocytes including natural killer (NK) and T cells. The extent of LIR-1 expression on NK cells is quite disparate between donors but the regulation of LIR-1 in NK cells is poorly understood. We examined expression profiles of LIR-1 on NK and T lymphocytes in 11 healthy donors over 1 year. Four of the 11 donors demonstrated substantial increases in LIR-1⁺ NK cells. High levels of LIR-1 expression were not correlated with exposure to human cytomegalovirus or the fraction of CD57⁺ NK cells in the donor. LIR-1 levels on ex vivo NK and CD56⁺ T cells were increased in vitro by short term exposure to IL-2 or IL-15 compared to control but not with various other cytokines tested. Sorted CD56(bright) NK cells also increased LIR-1 expression when cultured in IL-2. Maintenance of LIR-1 on longer term NK cells was also dependent on continuous stimulation by IL-15 or IL-2. While we could not detect increases in total LIR-1 mRNA in response to cytokine treatment by qPCR, we observed a shift in activity of LIR-1 promoter reporter constructs in the presence of IL-2 favoring the more translationally active transcript from the proximal promoter. Together these results show LIR-1 on NK cells is under the control of cytokines known to drive NK cell maturation and activation and suggest availability of such cytokines may alter the NK repertoire in vivo as we observed in several donors with fluctuating levels of LIR-1 on their NK cells.
Keywords: LIR-1; inhibitory receptor; natural killer cells.
Figures









References
-
- Anfossi N., Doisne J. M., Peyrat M. A., Ugolini S., Bonnaud O., Bossy D., Pitard V., Merville P., Moreau J. F., Delfraissy J. F., Dechanet-Merville J., Bonneville M., Venet A., Vivier E. (2004). Coordinated expression of Ig-like inhibitory MHC class I receptors and acquisition of cytotoxic function in human CD8+ T cells. J. Immunol. 173, 7223–7229 - PubMed
-
- Bellon T., Kitzig F., Sayos J., Lopez-Botet M. (2002). Mutational analysis of immunoreceptor tyrosine-based inhibition motifs of the Ig-like transcript 2 (CD85j) leukocyte receptor. J. Immunol. 168, 3351–3359 - PubMed
-
- Borges L., Hsu M. L., Fanger N., Kubin M., Cosman D. (1997). A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 159, 5192–5196 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

