Macrophages in synovial inflammation
- PMID: 22566842
- PMCID: PMC3342259
- DOI: 10.3389/fimmu.2011.00052
Macrophages in synovial inflammation
Abstract
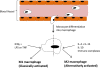
Synovial macrophages are one of the resident cell types in synovial tissue and while they remain relatively quiescent in the healthy joint, they become activated in the inflamed joint and, along with infiltrating monocytes/macrophages, regulate secretion of pro-inflammatory cytokines and enzymes involved in driving the inflammatory response and joint destruction. Synovial macrophages are positioned throughout the sub-lining layer and lining layer at the cartilage-pannus junction and mediate articular destruction. Sub-lining macrophages are now also considered as the most reliable biomarker for disease severity and response to therapy in rheumatoid arthritis (RA). There is a growing understanding of the molecular drivers of inflammation and an appreciation that the resolution of inflammation is an active process rather than a passive return to homeostasis, and this has implications for our understanding of the role of macrophages in inflammation. Macrophage phenotype determines the cytokine secretion profile and tissue destruction capabilities of these cells. Whereas inflammatory synovial macrophages have not yet been classified into one phenotype or another it is widely known that TNFα and IL-l, characteristically released by M1 macrophages, are abundant in RA while IL-10 activity, characteristic of M2 macrophages, is somewhat diminished. Here we will briefly review our current understanding of macrophages and macrophage polarization in RA as well as the elements implicated in controlling polarization, such as cytokines and transcription factors like NFκB, IRFs and NR4A, and pro-resolving factors, such as LXA4 and other lipid mediators which may promote a non-inflammatory, pro-resolving phenotype, and may represent a novel therapeutic paradigm.
Keywords: arthritis; inflammation; macrophage.
Figures
References
-
- Abdollahi-Roodsaz S., Joosten L. A. B., Koenders M. I., Devesa I., Roelofs M. F., Radstake T. R. D. J., Heuvelmans-Jacobs M., Akira S., Nicklin M. J. H., Ribeiro-Dias F. T., Van Den Berg W. B. (2008). Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–21610.1172/JCI32639 - DOI - PMC - PubMed
-
- Abeles A., Pillinger M. (2006). The role of the synovial fibroblast in rheumatoid arthritis: cartilage destruction and the regulation of matrix metalloproteinases. Bull. NYU. Hosp. Jt. Dis. 24, 20–24 - PubMed
-
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. (1990). Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J. Clin. Invest. 86, 1790–179810.1172/JCI114908 - DOI - PMC - PubMed
-
- Andrén M., Xiang Z., Nilsson G., Kleinau S. (2006). FcγRIII-expressing macrophages are essential for development of collagen-induced arthritis. Scand. J. Immunol. 63, 282–289 - PubMed
-
- Arur S., Uche U. E., Rezaul K., Fong M., Scranton V., Cowan A. E., Mohler W., Han D. K. (2003). Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 4, 587–598 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

