Tolerance and lymphoid organ structure and function
- PMID: 22566853
- PMCID: PMC3342028
- DOI: 10.3389/fimmu.2011.00064
Tolerance and lymphoid organ structure and function
Abstract
This issue of Frontiers in Immunologic Tolerance explores barriers to tolerance from a variety of views of cells, molecules, and processes of the immune system. Our laboratory has spent over a decade focused on the migration of the cells of the immune system, and dissecting the signals that determine how and where effector and suppressive regulatory T cells traffic from one site to another in order to reject or protect allografts. These studies have led us to a greater appreciation of the anatomic structure of the immune system, and the realization that the path taken by lymphocytes during the course of the immune response to implanted organs determines the final outcome. In particular, the structures, microanatomic domains, and the cells and molecules that lymphocytes encounter during their transit through blood, tissues, lymphatics, and secondary lymphoid organs are powerful determinants for whether tolerance is achieved. Thus, the understanding of complex cellular and molecular processes of tolerance will not come from "96-well plate immunology," but from an integrated understanding of the temporal and spatial changes that occur during the response to the allograft. The study of the precise positioning and movement of cells in lymphoid organs has been difficult since it is hard to visualize cells within their three-dimensional setting; instead techniques have tended to be dominated by two-dimensional renderings, although advanced confocal and two-photon systems are changing this view. It is difficult to precisely modify key molecules and events in lymphoid organs, so that existing knockouts, transgenics, inhibitors, and activators have global and pleiotropic effects, rather than precise anatomically restricted influences. Lastly, there are no well-defined postal codes or tracking systems for leukocytes, so that while we can usually track cells from point A to point B, it is exponentially more difficult or even impossible to track them to point C and beyond. We believe this represents one of the fundamental barriers to understanding the immune system and devising therapeutic approaches that take into account anatomy and structure as major controlling principles of tolerance.
Keywords: lymph node; structure; tolerance.
Figures

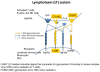
References
-
- Alferink J., Lieberam I., Reindl W., Behrens A., Weiss S., Huser N., Gerauer K., Ross R., Reske-Kunz A. B., Ahmad-Nejad P., Wagner H., Forster I. (2003). Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J. Exp. Med. 197, 585–59910.1084/jem.20021859 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

