Antibodies against conserved antigens provide opportunities for reform in influenza vaccine design
- PMID: 22566865
- PMCID: PMC3342000
- DOI: 10.3389/fimmu.2011.00076
Antibodies against conserved antigens provide opportunities for reform in influenza vaccine design
Abstract
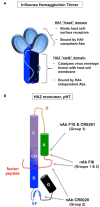
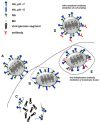
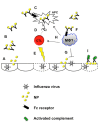
High-performance neutralizing antibody against influenza virus typically recognizes the globular head region of its hemagglutinin (HA) envelope glycoprotein. To-date, approved human vaccination strategies have been designed to induce such antibodies as a sole means of preventing the consequences of this infection. However, frequent amino-acid changes in the HA globular head allow for efficient immune evasion. Consequently, vaccines inducing such neutralizing antibodies need to be annually re-designed and re-administered at a great expense. These vaccines furthermore provide little-to-no immunity against antigenic-shift strains, which arise from complete replacement of HA or of neuraminidase genes, and pose pandemic risks. To address these issues, laboratory research has focused on inducing immunity effective against all strains, regardless of changes in the HA globular head. Despite prior dogma that such cross-protection needs to be induced by cellular immunity alone, several advances in recent years demonstrate that antibodies of other specificities are capable of cross-strain protection in mice. This review discusses the reactivity, induction, efficacy, and mechanisms of antibodies that react with poorly accessible epitopes in the HA stalk, with the matrix 2 membrane ion channel, and even with the internal nucleoprotein. These advances warrant further investigation of the inducibility and efficacy of such revolutionary antibody strategies in humans.
Keywords: antibody; hemagglutinin; influenza virus; matrix 2 external domain; nucleoprotein; vaccine.
Figures

References
-
- (1989). Oxford English Dictionary. Available at: http://www.oed.com/view/Entry/95531?redirectedFrom=influenza#eid
-
- (2011). Review of the 2010-2011 winter influenza season, northern hemisphere. Wkly. Epidemiol. Rec. 86, 222–227 - PubMed
-
- Ahmad R., Sindhu S. T., Toma E., Morisset R., Vincelette J., Menezes J., Ahmad A. (2001). Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21, 227–23310.1023/A:1011087132180 - DOI - PubMed
-
- Beran J., Vesikari T., Wertzova V., Karvonen A., Honegr K., Lindblad N., Van Belle P., Peeters M., Innis B. L., Devaster J. M. (2009). Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J. Infect. Dis. 200, 1861–186910.1086/648406 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

