The EMILIN/Multimerin family
- PMID: 22566882
- PMCID: PMC3342094
- DOI: 10.3389/fimmu.2011.00093
The EMILIN/Multimerin family
Abstract
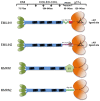
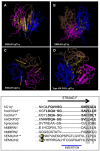
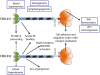
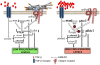
Elastin microfibrillar interface proteins (EMILINs) and Multimerins (EMILIN1, EMILIN2, Multimerin1, and Multimerin2) constitute a four member family that in addition to the shared C-terminus gC1q domain typical of the gC1q/TNF superfamily members contain a N-terminus unique cysteine-rich EMI domain. These glycoproteins are homotrimeric and assemble into high molecular weight multimers. They are predominantly expressed in the extracellular matrix and contribute to several cellular functions in part associated with the gC1q domain and in part not yet assigned nor linked to other specific regions of the sequence. Among the latter is the control of arterial blood pressure, the inhibition of Bacillus anthracis cell cytotoxicity, the promotion of cell death, the proangiogenic function, and a role in platelet hemostasis. The focus of this review is to highlight the multiplicity of functions and domains of the EMILIN/Multimerin family with a particular emphasis on the regulatory role played by the ligand-receptor interactions of the gC1q domain. EMILIN1 is the most extensively studied member both from the structural and functional point of view. The structure of the gC1q of EMILIN1 solved by NMR highlights unique characteristics compared to other gC1q domains: it shows a marked decrease of the contact surface of the trimeric assembly and while conserving the jelly-roll topology with two β-sheets of antiparallel strands it presents a nine-stranded β-sandwich fold instead of the usual 10-stranded fold. This is likely due to the insertion of nine residues that disrupt the ordered strand organization and forma a highly dynamic protruding loop. In this loop the residue E933 is the site of interaction between gC1q and the α4β1 and α9β1 integrins, and contrary to integrin occupancy that usually upregulates cell growth, when gC1q is ligated by the integrin the cells reduce their proliferative activity.
Keywords: EMI domain; cell migration; extracellular matrix; gC1q NMR solution structure; gC1q-dependent cell adhesion; skin homeostasis; α4β1 integrin.
Figures
References
-
- Adam F., Zheng S., Joshi N., Kelton D. S., Sandhu A., Suehiro Y., Jeimy S. B., Santos A. V., Massé J. M., Kelton J. G., Cramer E. M., Hayward C. P. (2005). Analyses of cellular multimerin 1 receptors: in vitro evidence of binding mediated by alphaIIbbeta3 and alphavbeta3. Thromb. Haemost. 94, 1004–1011 - PubMed
-
- Ahuia N., Kumar P., Alam S., Gupta M., Bhatnagar R. (2003). Deletion mutants of protective antigen that inhibit anthrax toxin both in vitro and in vivo. Biochem. Biophys. Res. Commun. 37, 446–450 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

