Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44
- PMID: 22566907
- PMCID: PMC3342067
- DOI: 10.3389/fimmu.2012.00023
Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44
Abstract
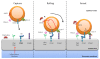
Despite the widespread use of the cell-surface receptor CD44 as a marker for antigen (Ag)-experienced, effector and memory T cells, surprisingly little is known regarding its function on these cells. The best-established function of CD44 is the regulation of cell adhesion and migration. As such, the interactions of CD44, primarily with its major ligand, the extracellular matrix (ECM) component hyaluronic acid (HA), can be crucial for the recruitment and function of effector and memory T cells into/within inflamed tissues. However, little is known about the signaling events following engagement of CD44 on T cells and how cooperative interactions of CD44 with other surface receptors affect T cell responses. Recent evidence suggests that the CD44 signaling pathway(s) may be shared with those of other adhesion receptors, and that these provide contextual signals at different anatomical sites to ensure the correct T cell effector responses. Furthermore, CD44 ligation may augment T cell activation after Ag encounter and promote T cell survival, as well as contribute to regulation of the contraction phase of an immune response and the maintenance of tolerance. Once the memory phase is established, CD44 may have a role in ensuring the functional fitness of memory T cells. Thus, the summation of potential signals after CD44 ligation on T cells highlights that migration and adhesion to the ECM can critically impact the development and homeostasis of memory T cells, and may differentially affect subsets of T cells. These aspects of CD44 biology on T cells and how they might be modulated for translational purposes are discussed.
Keywords: CD44; T cell; extracellular matrix; memory; migration.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

