Molecular mechanisms of treg-mediated T cell suppression
- PMID: 22566933
- PMCID: PMC3341960
- DOI: 10.3389/fimmu.2012.00051
Molecular mechanisms of treg-mediated T cell suppression
Abstract
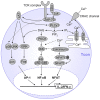
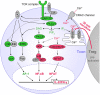
CD4(+)CD25(high)Foxp3(+) regulatory T cells (Tregs) can suppress other immune cells and, thus, are critical mediators of peripheral self-tolerance. On the one hand, Tregs avert autoimmune disease and allergies. On the other hand, Tregs can prevent immune reactions against tumors and pathogens. Despite the importance of Tregs, the molecular mechanisms of suppression remain incompletely understood and controversial. Proliferation and cytokine production of CD4(+)CD25(-) conventional T cells (Tcons) can be inhibited directly by Tregs. In addition, Tregs can indirectly suppress Tcon activation via inhibition of the stimulatory capacity of antigen presenting cells. Direct suppression of Tcons by Tregs can involve immunosuppressive soluble factors or cell contact. Different mechanisms of suppression have been described, so far with no consensus on one universal mechanism. Controversies might be explained by the fact that different mechanisms may operate depending on the site of the immune reaction, on the type and activation state of the suppressed target cell as well as on the Treg activation status. Further, inhibition of T cell effector function can occur independently of suppression of proliferation. In this review, we summarize the described molecular mechanisms of suppression with a particular focus on suppression of Tcons and rapid suppression of T cell receptor-induced calcium (Ca(2+)), NFAT, and NF-κB signaling in Tcons by Tregs.
Keywords: TCR signaling; Treg; suppression mechanisms.
Figures

References
-
- Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Knight G. E., Fumagalli M., Gachet C., Jacobson K. A., Weisman G. A. (2006). International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–34110.1124/pr.58.3.3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

