Key Features of the Intragraft Microenvironment that Determine Long-Term Survival Following Transplantation
- PMID: 22566935
- PMCID: PMC3342046
- DOI: 10.3389/fimmu.2012.00054
Key Features of the Intragraft Microenvironment that Determine Long-Term Survival Following Transplantation
Abstract
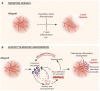
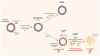
In this review, we discuss how changes in the intragraft microenvironment serve to promote or sustain the development of chronic allograft rejection. We propose two key elements within the microenvironment that contribute to the rejection process. The first is endothelial cell proliferation and angiogenesis that serve to create abnormal microvascular blood flow patterns as well as local tissue hypoxia, and precedes endothelial-to-mesenchymal transition. The second is the overexpression of local cytokines and growth factors that serve to sustain inflammation and, in turn, function to promote a leukocyte-induced angiogenesis reaction. Central to both events is overexpression of vascular endothelial growth factor (VEGF), which is both pro-inflammatory and pro-angiogenic, and thus drives progression of the chronic rejection microenvironment. In our discussion, we focus on how inflammation results in angiogenesis and how leukocyte-induced angiogenesis is pathological. We also discuss how VEGF is a master control factor that fosters the development of the chronic rejection microenvironment. Overall, this review provides insight into the intragraft microenvironment as an important paradigm for future direction in the field.
Keywords: allograft rejection; allograft vasculopathy; angiogenesis; chronic allograft rejection; endothelial cell; hypoxia; microvascular injury; vascular endothelial growth factor.
Figures
References
-
- Abramson L. P., Pahl E., Huang L., Stellmach V., Rodgers S., Mavroudis C., Backer C. L., Arensman R. M., Crawford S. E. (2002). Serum vascular endothelial growth factor as a surveillance marker for cellular rejection in pediatric cardiac transplantation. Transplantation 73, 153–15610.1097/00007890-200201150-00030 - DOI - PubMed
-
- Arciniegas E., Sutton A. B., Allen T. D., Schor A. M. (1992). Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J. Cell. Sci. 103(Pt 2), 521–529 - PubMed
-
- Arora S., Ueland T., Wennerblom B., Sigurdadottir V., Eiskjaer H., Botker H. E., Ekmehag B., Jansson K., Mortensen S. A., Saunamaki K., Simonsen S., Gude E., Bendz B., Solbu D., Aukrust P., Gullestad L. (2011). Effect of everolimus introduction on cardiac allograft vasculopathy – results of a randomized, multicenter trial. Transplantation 92, 235–24310.1097/TP.0b013e31822057f1 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

