The role of heat shock proteins in antigen cross presentation
- PMID: 22566944
- PMCID: PMC3342350
- DOI: 10.3389/fimmu.2012.00063
The role of heat shock proteins in antigen cross presentation
Abstract
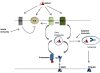
Heat shock proteins (HSPs) are molecular chaperones that bind tumor antigens and mediate their uptake into antigen presenting cells. HSP-antigen complexes are then directed toward either the MHC class I pathway through antigen cross presentation or the conventional class II pathway, leading to activation of T cell subsets. Uptake of HSP-chaperoned polypeptides can involve both receptor-mediated and receptor-independent routes, and mechanisms of antigen sorting between the Class I and II pathways after uptake are currently under investigation. The processes involved in internalization of HSP-antigen complexes differ somewhat from the mechanisms previously determined for (unchaperoned) particulate and free soluble antigens. A number of studies show that HSP-facilitated antigen cross presentation requires uptake of the complexes by scavenger receptors (SR) followed by processing in the proteasome, and loading onto MHC class I molecules. In this review we have examined the roles of HSPs and SR in antigen uptake, sorting, processing, cell signaling, and activation of innate and adaptive immunity.
Keywords: CTL response; anti-cancer vaccine; antigen cross presentation; antigen presenting cells; heat shock proteins; scavenger receptor; soluble vs. particulate antigen; tumor immunity.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

