Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis
- PMID: 22566952
- PMCID: PMC3342381
- DOI: 10.3389/fimmu.2012.00071
Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis
Abstract
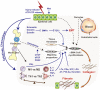
Organ fibrosis is a pathological condition associated with chronic inflammatory diseases. In fibrosis, excessive deposition of extracellular matrix (ECM) severely impairs tissue architecture and function, eventually resulting in organ failure. This process is mediated primarily by the induction of myofibroblasts, which produce large amounts of collagen I, the main component of the ECM. Accordingly, the origin, developmental pathways, and mechanisms of myofibroblast regulation are attracting increasing attention as potential therapeutic targets. The fibrotic cascade, from initial epithelial damage to eventual myofibroblast induction, is mediated by complex biological processes such as macrophage infiltration, a shift from Th1 to Th2 phenotype, and by inflammatory mediators such as transforming growth factor-β. Here, we review the current understanding of the cellular and molecular mechanisms underlying organ fibrosis.
Keywords: TGFb; chemokine; collagen I; fibroblast; fibrosis; mesenchymal stem cell; myofibroblast; pericyte.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

