Inflammation and bone destruction in arthritis: synergistic activity of immune and mesenchymal cells in joints
- PMID: 22566958
- PMCID: PMC3342288
- DOI: 10.3389/fimmu.2012.00077
Inflammation and bone destruction in arthritis: synergistic activity of immune and mesenchymal cells in joints
Abstract
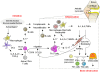
Rheumatoid arthritis (RA) is an immune-mediated disease of the joints that is characterized by chronic inflammation and synovial hyperplasia that eventually lead to cartilage and bone destruction. Synovial fibroblasts are mesenchymal cells recognized as a key cell population in RA due to their hyperproliferative and hypersensitive properties in the inflammatory milieu and hyperproduction of both inflammatory cytokines and matrix-degrading enzymes. On the immune cell side, a wealth of evidence has shown that CD4(+)T-cells, especially IL-17 producing helper T (Th17) cells, play a prominent role, particularly in the initiation of systemic immune response in RA. However, it is still unclear how the local chronic inflammation in the joint is elicited by a systemic immune response. Recent studies have shed light on the importance of the interaction between immune and mesenchymal cells in joints including synovial fibroblasts. In particular, mesenchymal cells contribute to the Th17-mediated chronic inflammation in RA by promoting the migration of Th17 cells to the inflamed site and then the homeostatic proliferation and concomitant increase in IL-17 production. In addition, recent progress in osteoimmunology has provided new insight into the pathogenesis of the bone destruction which takes place in RA. Th17-related cytokines have been shown to enhance osteoclastogenesis, mainly via synovial fibroblasts. Thus, mesenchymal cells are a determinant of the development of RA that links the systemic immune response and the local disorder in the joints. In addition, the interaction of immune and mesenchymal cells plays a key role in both the chronic inflammation and bone destruction seen in RA. Elucidation of the precise events involved in this interaction will lead to a better understanding of the mechanisms by which chronic inflammation and bone destruction in joint results from a systemic immune response, and also will help provide a molecular basis for novel therapeutic strategies to treat RA.
Keywords: CD4+T-cell; Th17 cell; bone destruction; inflammation; osteoclast; rheumatoid arthritis; synovial fibroblast.
Figures
References
-
- Abdollahi-Roodsaz S., Joosten L. A., Koenders M. I., Devesa I., Roelofs M. F., Radstake T. R., Heuvelmans-Jacobs M., Akira S., Nicklin M. J., Ribeiro-Dias F., van Den Berg W. B. (2008). Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–21610.1172/JCI32639 - DOI - PMC - PubMed
-
- Abdollahi-Roodsaz S., Joosten L. A., Roelofs M. F., Radstake T. R., Matera G., Popa C., Van Der Meer J. W., Netea M. G., Van Den Berg W. B. (2007). Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–296710.1002/art.22848 - DOI - PubMed
-
- Asagiri M., Hirai T., Kunigami T., Kamano S., Gober H. J., Okamoto K., Nishikawa K., Latz E., Golenbock D. T., Aoki K., Ohya K., Imai Y., Morishita Y., Miyazono K., Kato S., Saftig P., Takayanagi H. (2008). Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science 319, 624–62710.1126/science.1150110 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

