Salivaricin G32, a Homolog of the Prototype Streptococcus pyogenes Nisin-Like Lantibiotic SA-FF22, Produced by the Commensal Species Streptococcus salivarius
- PMID: 22567013
- PMCID: PMC3332205
- DOI: 10.1155/2012/738503
Salivaricin G32, a Homolog of the Prototype Streptococcus pyogenes Nisin-Like Lantibiotic SA-FF22, Produced by the Commensal Species Streptococcus salivarius
Abstract
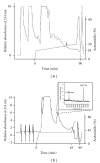
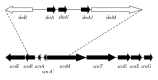
Salivaricin G32, a 2667 Da novel member of the SA-FF22 cluster of lantibiotics, has been purified and characterized from Streptococcus salivarius strain G32. The inhibitory peptide differs from the Streptococcus pyogenes-produced SA-FF22 in the absence of lysine in position 2. The salivaricin G32 locus was widely distributed in BLIS-producing S. salivarius, with 6 (23%) of 26 strains PCR-positive for the structural gene, slnA. As for most other lantibiotics produced by S. salivarius, the salivaricin G32 locus can be megaplasmid encoded. Another member of the SA-FF22 family was detected in two Streptococcus dysgalactiae of bovine origin, an observation supportive of widespread distribution of this lantibiotic within the genus Streptococcus. Since the inhibitory spectrum of salivaricin G32 includes Streptococcus pyogenes, its production by S. salivarius, either as a member of the normal oral microflora or as a commercial probiotic, could serve to enhance protection of the human host against S. pyogenes infection.
Figures


Similar articles
-
Prevention of streptococcal pharyngitis by anti-Streptococcus pyogenes bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius.Indian J Med Res. 2004 May;119 Suppl:13-6. Indian J Med Res. 2004. PMID: 15232154
-
Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius.Microbiology (Reading). 2011 May;157(Pt 5):1290-1299. doi: 10.1099/mic.0.044719-0. Epub 2011 Feb 10. Microbiology (Reading). 2011. PMID: 21310787
-
Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12.Appl Environ Microbiol. 2007 Feb;73(4):1107-13. doi: 10.1128/AEM.02265-06. Epub 2006 Dec 28. Appl Environ Microbiol. 2007. PMID: 17194838 Free PMC article.
-
Bacteriocin Production by Beta-Hemolytic Streptococci.Pathogens. 2021 Jul 9;10(7):867. doi: 10.3390/pathogens10070867. Pathogens. 2021. PMID: 34358017 Free PMC article. Review.
-
Evolution of Lantibiotic Salivaricins: New Weapons to Fight Infectious Diseases.Trends Microbiol. 2020 Jul;28(7):578-593. doi: 10.1016/j.tim.2020.03.001. Epub 2020 Apr 6. Trends Microbiol. 2020. PMID: 32544444 Review.
Cited by
-
Tackling Multidrug Resistance in Streptococci - From Novel Biotherapeutic Strategies to Nanomedicines.Front Microbiol. 2020 Oct 6;11:579916. doi: 10.3389/fmicb.2020.579916. eCollection 2020. Front Microbiol. 2020. PMID: 33123110 Free PMC article. Review.
-
HUMAN MICROBIOTA. Small molecules from the human microbiota.Science. 2015 Jul 24;349(6246):1254766. doi: 10.1126/science.1254766. Epub 2015 Jul 23. Science. 2015. PMID: 26206939 Free PMC article. Review.
-
Virgicin, a novel lanthipeptide from Virgibacillus sp. strain AK90 exhibits inhibitory activity against Gram-positive bacteria.World J Microbiol Biotechnol. 2019 Aug 20;35(9):133. doi: 10.1007/s11274-019-2707-9. World J Microbiol Biotechnol. 2019. PMID: 31432254
-
Beneficial modulation of human health in the oral cavity and beyond using bacteriocin-like inhibitory substance-producing streptococcal probiotics.Front Microbiol. 2023 Mar 28;14:1161155. doi: 10.3389/fmicb.2023.1161155. eCollection 2023. Front Microbiol. 2023. PMID: 37056747 Free PMC article. Review.
-
The Genomic Characteristics of Potential Probiotics: Two Streptococcus salivarius Isolates from a Healthy Individual in China.Microorganisms. 2025 Mar 20;13(3):694. doi: 10.3390/microorganisms13030694. Microorganisms. 2025. PMID: 40142586 Free PMC article.
References
-
- Jack RW, Carne A, Metzger J, et al. Elucidation of the structure of SA-FF22, a lanthionine-containing antibacterial peptide produced by Streptococcus pyogenes strain FF22. European Journal of Biochemistry. 1994;220(2):455–462. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

