Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-κB and upregulation of IL-1Ra in melanoma model
- PMID: 22567084
- PMCID: PMC3342329
- DOI: 10.1371/journal.pone.0033747
Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-κB and upregulation of IL-1Ra in melanoma model
Abstract
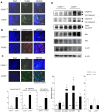
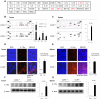
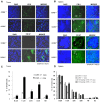
To evaluate the relevance of C-C chemokine receptor type 5 (CCR5) expression and tumor development, we compared melanoma growth in CCR5 knockout (CCR5(-/-)) mice and wild type (CCR5(+/+)) mice. CCR5(-/-) mice showed reduced tumor volume, tumor weight, and increased survival rate when compared to CCR5(+/+) mice. We investigated the activation of NF-κB since it is an implicated transcription factor in the regulation of genes involving cell growth, apoptosis, and tumor growth. Significant inhibition of DNA binding activity of NF-κB, and translocation of p50 and p65 into the nucleus through the inhibition of phosphorylation of IκB was found in the melanoma tissues of CCR5(-/-) mice compared to melanoma tissues of CCR5(+/+) mice. NF-κB target apoptotic protein expression, such as cleaved caspase-3, cleaved PARP, and Bax, was elevated, whereas the survival protein expression levels, such as Bcl-2, C-IAP1, was decreased in the melanoma tissues of CCR5(-/-) mice. Interestingly, we found that the level of IL-1Ra, a tumor growth suppressive cytokine, was significantly elevated in tumor tissue and spleen of CCR5(-/-) mice compared to the level in CCR5(+/+) mice. Moreover, infiltration of CD8(+) cytotoxic T cell and CD57(+) natural killer cells was significantly increased in melanoma tumor and spleen tissue of CCR5(-/-) mice compared to that of CCR5(+/+) mice. Therefore, these results showed that CCR5 deficiency caused apoptotic cell death of melanoma through inhibition of NF-κB and upregulation of IL-1Ra.
Conflict of interest statement
Figures


References
-
- Wu X, Lee VC, Chevalier E, Hwang ST. Chemokine receptors as targets for cancer therapy. Curr Pharm Des. 2009;15:742–757. - PubMed
-
- Erreni M, Bianchi P, Laghi L, Mirolo M, Fabbri M, et al. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009;460:105–121. - PubMed
-
- Seidl H, Richtig E, Tilz H, Stefan M, Schmidbauer U, et al. Profiles of chemokine receptors in melanocytic lesions: de novo expression of CXCR6 in melanoma. Hum Pathol. 2007;38:768–780. - PubMed
-
- Sutton A, Friand V, Brule-Donneger S, Chaigneau T, Ziol M, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. - PubMed
-
- Aldinucci D, Lorenzon D, Cattaruzza L, Pinto A, Gloghini A, et al. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer. 2008;122:769–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

