Efficacy of memantine for agitation in Alzheimer's dementia: a randomised double-blind placebo controlled trial
- PMID: 22567095
- PMCID: PMC3342281
- DOI: 10.1371/journal.pone.0035185
Efficacy of memantine for agitation in Alzheimer's dementia: a randomised double-blind placebo controlled trial
Abstract
Background: Agitation in Alzheimer's disease (AD) is common and associated with poor patient life-quality and carer distress. The best evidence-based pharmacological treatments are antipsychotics which have limited benefits with increased morbidity and mortality. There are no memantine trials in clinically significant agitation but post-hoc analyses in other populations found reduced agitation. We tested the primary hypothesis, memantine is superior to placebo for clinically significant agitation, in patients with moderate-to-severe AD.
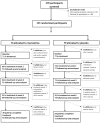
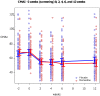
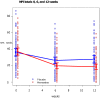
Methods and findings: We recruited 153 participants with AD and clinically significant agitation from care-homes or hospitals for a double-blind randomised-controlled trial and 149 people started the trial of memantine versus placebo. The primary outcome was 6 weeks mixed model autoregressive analysis of Cohen-Mansfield Agitation Inventory (CMAI). Secondary outcomes were: 12 weeks CMAI; 6 and 12 weeks Neuropsychiatric symptoms (NPI), Clinical Global Impression Change (CGI-C), Standardised Mini Mental State Examination, Severe Impairment Battery. Using a mixed effects model we found no significant differences in the primary outcome, 6 weeks CMAI, between memantine and placebo (memantine lower -3.0; -8.3 to 2.2, p = 0.26); or 12 weeks CMAI; or CGI-C or adverse events at 6 or 12 weeks. NPI mean difference favoured memantine at weeks 6 (-6.9; -12.2 to -1.6; p = 0.012) and 12 (-9.6; -15.0 to -4.3 p = 0.0005). Memantine was significantly better than placebo for cognition. The main study limitation is that it still remains to be determined whether memantine has a role in milder agitation in AD.
Conclusions: Memantine did not improve significant agitation in people with in moderate-to-severe AD. Future studies are urgently needed to test other pharmacological candidates in this group and memantine for neuropsychiatric symptoms.
Trial registration: ClinicalTrials.gov NCT00371059.
Trial registration: International Standard Randomised Controlled Trial 24953404.
Conflict of interest statement
Figures
References
-
- Wilmo A, Prince M. The global economic impact of dementia. London; 2010; 2010. World Alzheimer’s Report 2010.
-
- Ryu SH, Katona C, Rive B, Livingston G. Persistence of and changes in neuropsychiatric symptoms in Alzheimer disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry. 13 (! 2005;1):976–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical