Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled trial
- PMID: 22567287
- PMCID: PMC3337486
- DOI: 10.1155/2012/452541
Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled trial
Abstract
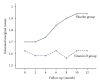
The aim of this preliminary study was to evaluate the effect of low-dose oral vitamin D in combination with current disease-modifying therapy on the prevention of progression of relapsing-remitting multiple sclerosis (RRMS). A phase II double-blind placebo-controlled randomized clinical trial conducted between October 2007 and October 2008 included 50 patients with confirmed RRMS aged 25 to 57 years and normal serum 25-hydroxyvitamin D. They were randomly allocated to receive 12 months of treatment with either escalating calcitriol doses up to 0.5 μg/day or placebo combined with disease-modifying therapy. Response to treatment was assessed at eight-week intervals. In both groups, the mean relapse rate decreased significantly (P < 0.001). In the 25 patients treated with placebo, the mean (SD) Expanded Disability Status Scale (EDSS) increased from 1.70 (1.21) at baseline to 1.94 (1.41) at the end of study period (P < 0.01). Average EDSS and relapse rate at the end of trial did not differ between groups. Adding low-dose vitamin D to routine disease-modifying therapy had no significant effect on the EDSS score or relapse rate. A larger phase III multicenter study of vitamin D in RRMS is warranted to more assess the efficacy of this intervention.
Figures
References
-
- Clanet MG, Brassat D. The management of multiple sclerosis patients. Current Opinion in Neurology. 2000;13(3):263–270. - PubMed
-
- Richards RG, Sampson FC, Beard SM, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technology Assessment. 2002;6(10):1–73. - PubMed
-
- Davis WM. Multiple sclerosis: continuing mysteries and current management. Drug Topics. 2000;144(12):93–102.
-
- Miller A. Current and investigational therapies used to alter the course of disease in multiple sclerosis. Southern Medical Journal. 1997;90(4):367–375. - PubMed
LinkOut - more resources
Full Text Sources
Medical