Vancomycin-associated leukocytoclastic vasculitis
- PMID: 22567469
- PMCID: PMC3336219
- DOI: 10.1155/2011/356370
Vancomycin-associated leukocytoclastic vasculitis
Abstract
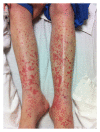
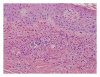
Vancomycin is U.S. Food and Drug Administration (FDA) approved for treatment of serious infections caused by methicillin-resistant Staphylococcus aureus (MRSA) or in individuals who have failed, cannot tolerate, or are allergic to other antibiotics. Very few cases of vancomycin-associated leukocytoclastic vasculitis have been published. We report on a patient who developed pruritus and palpable purpura in both lower extremities after receiving six days of intravenous vancomycin. Skin biopsy revealed leukocytoclastic vasculitis.
Figures
References
-
- Ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Annals of Pharmacotherapy. 2002;36(1):130–147. - PubMed
-
- Fujikawa K, Kawakami A, Hayashi T, et al. Cutaneous vasculitis induced by TNF inhibitors: a report of three cases. Modern Rheumatology. 2010;20(1):86–89. - PubMed
-
- Solky BA, Pincus L, Horan RF. Vancomycin-induced linear IgA bullous dermatosis: morphology is a key to diagnosis. Cutis. 2004;73(1):65–67. - PubMed
-
- Senanayake SN, Hardman DT, Miller AC. Case of vancomycin-induced linear Immunoglobulin A bullous dermatosis. Internal Medicine Journal. 2008;38(7):p. 607. - PubMed
-
- Whitworth JM, Thomas I, Peltz SA, Sullivan BC, Wolf AH, Cytryn AS. Vancomycin-induced linear IgA bullous dermatosis (LABD) Journal of the American Academy of Dermatology. 1996;34(5):890–891. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

