Improving optical contact for functional near‑infrared brain spectroscopy and imaging with brush optodes
- PMID: 22567582
- PMCID: PMC3342194
- DOI: 10.1364/BOE.3.000878
Improving optical contact for functional near‑infrared brain spectroscopy and imaging with brush optodes
Abstract
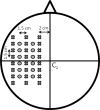
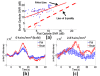
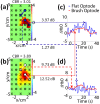
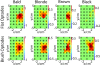
A novel brush optode was designed and demonstrated to overcome poor optical contact with the scalp that can occur during functional near infrared spectroscopy (fNIRS) and imaging due to light obstruction by hair. The brush optodes were implemented as an attachment to existing commercial flat-faced (conventional) fiber bundle optodes. The goal was that the brush optodes would thread through hair and improve optical contact on subjects with dense hair. Simulations and experiments were performed to assess the magnitude of these improvements. FNIRS measurements on 17 subjects with varying hair colors (blonde, brown, and black) and hair densities (0-2.96 hairs/mm(2)) were performed during a finger tapping protocol for both flat and brush optodes. In addition to reaching a study success rate of almost 100% when using the brush optode extensions, the measurement setup times were reduced by a factor of three. Furthermore, the brush optodes enabled improvements in the activation signal-to-noise ratio (SNR) by up to a factor of ten as well as significant (p < 0.05) increases in the detected area of activation (dAoA). The measured improvements in SNR were matched by Monte Carlo (MC) simulations of photon propagation through scalp and hair. In addition, an analytical model was derived to mathematically estimate the observed light power losses due to different hair colors and hair densities. Interestingly, the derived analytical formula produced excellent estimates of the experimental data and MC simulation results despite several simplifying assumptions. The analytical model enables researchers to readily estimate the light power losses due to obstruction by hair for both flat-faced fiber bundles and individual fibers for a given subject.
Keywords: (170.2655) Functional monitoring and imaging; (170.3880) Medical and biological imaging; (300.6340) Spectroscopy, infrared.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
