Novel organotypic culture model of cholangiocarcinoma progression
- PMID: 22568523
- PMCID: PMC3416930
- DOI: 10.1111/j.1872-034X.2012.01026.x
Novel organotypic culture model of cholangiocarcinoma progression
Abstract
Aim: Recent studies have suggested that increased α-smooth muscle-actin positive myofibroblastic cells (α-SMA positive CAF) in the desmoplastic stroma may relate to a more aggressive cancer and worse survival outcomes for intrahepatic cholangiocarcinoma (ICC) patients. To facilitate investigating cellular and molecular interactions between α-SMA positive CAF and cholangiocarcinoma cells related to ICC progression, we developed a novel 3-D organotypic culture model of cholangiocarcinoma that more accurately mimics the stromal microenvironment, gene expression profile and select pathophysiological characteristics of desmoplastic ICC in vivo.
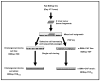
Methods: This unique model was established by co-culturing within a type I collagen gel matrix, a strain of cholangiocarcinoma cells (derived from an ICC formed in syngeneic rat liver following bile duct inoculation of spontaneously-transformed rat cholangiocytes) with varying numbers of clonal α-SMA positive CAF established from the same tumor type.
Results: Cholangiocarcinoma cells and α-SMA positive CAF in monoculture each exhibited cell-specific biomarker gene expression profiles characteristic of stromal myofibroblastic cell versus malignant cholangiocyte cell types. In comparison, the gene expression profile and histopathological characteristics exhibited by the organotypic co-culture closely resembled those of whole tissue samples of the parent orthotopic ICC. We further showed α-SMA positive CAF to significantly enhance cholangiocarcinoma cell "ductal-like" growth and cancer cell migration/invasiveness in vitro, as well as to promote upregulated expression of select genes known to be associated with ICC invasion.
Conclusion: This novel organotypic model provides an important new resource for studying the effects of microenvironment on cholangiocarcinoma progression in vitro and may have potential as a preclinical model for identifying molecularly targeted therapies.
© 2012 The Japan Society of Hepatology.
Conflict of interest statement
Figures





 = mean ± SD) was determined to be significantly greater at the higher initial BDEsp-TDFE4 cell plating densities. (E) Representative data demonstrating BDEsp-TDFE4 CAFsto increase the cell migration/invasiveness of BDEsp-TDEH10 cholangiocarcinoma cells in vitro when assessed in the Matrigel™ invasion bioassay system. T= top chamber coated by Matrigel; B = bottom culture well coated with rat tail type I collagen. Invasion Index was determined according to the manufacturer’s instructions.
= mean ± SD) was determined to be significantly greater at the higher initial BDEsp-TDFE4 cell plating densities. (E) Representative data demonstrating BDEsp-TDFE4 CAFsto increase the cell migration/invasiveness of BDEsp-TDEH10 cholangiocarcinoma cells in vitro when assessed in the Matrigel™ invasion bioassay system. T= top chamber coated by Matrigel; B = bottom culture well coated with rat tail type I collagen. Invasion Index was determined according to the manufacturer’s instructions.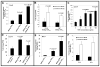
References
-
- Terada T, Makimoto K, Terayama N, et al. Alpha-smooth muscle actin-positive stromal cells in cholangiocarcinomas, hepatocellular carcinomas and metastatic liver carcinomas. J Hepatol. 1996;24:706–712. - PubMed
-
- Okamura N, Yoshida M, Shibuya A, et al. Cellular and stromal characteristics in the scirrhous hepatocellular carcinoma: comparison with hepatocellular carcinomas and intrahepatic cholangiocarcinomas. Pathol Int. 2005;55:724–731. - PubMed
-
- Sirica AE, Campbell DJ, Dumur CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2011;27:276–284. - PubMed
-
- Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

