Altered regulation of CXCR4 expression during aging contributes to increased CXCL12-dependent chemotactic migration of CD4(+) T cells
- PMID: 22568557
- PMCID: PMC3399962
- DOI: 10.1111/j.1474-9726.2012.00830.x
Altered regulation of CXCR4 expression during aging contributes to increased CXCL12-dependent chemotactic migration of CD4(+) T cells
Abstract
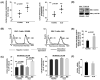
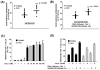
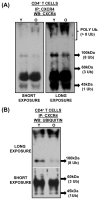
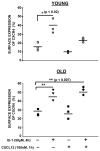
Chemokine-dependent migration of T lymphocytes assures recirculation of naïve T cells to secondary lymphoid organs and tissue-specific trafficking of memory-effector T cells. Previous studies carried out in rodents have demonstrated age-associated modulation of the expression of chemokine receptors such as CXCR4 and CCR5; however, little is known about the molecular mechanisms that regulate receptor expression and turnover in T cells, during advancing age in humans. Our recent results demonstrating increased chemotactic migration in response to CXCL12 in CD4(+) T cells obtained from the elderly, as compared to those from young donors, led us to hypothesize that increase in surface expression, because of altered endocytic regulation of CXCR4 on T cells during aging, might be directly responsible for increased migration toward CXCL12. Studies presented here demonstrate a significant increase in the surface expression of CXCR4 in CD4(+) T cells from elderly human donors, relative to those from the young. Additionally, CXCL12-mediated endocytosis of CXCR4 was differentially regulated during aging, which could be attributed to alterations in the ubiquitination of CXCR4. Thus, altered ubiquitination of CXCR4 may contribute to the increased surface expression and enhanced T-cell migration to chemotactic stimuli in the elderly.
© 2012 The Authors. Aging Cell © 2012 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures
Similar articles
-
Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation.J Biol Chem. 2009 Feb 27;284(9):5742-52. doi: 10.1074/jbc.M808507200. Epub 2008 Dec 23. J Biol Chem. 2009. PMID: 19106094 Free PMC article.
-
Coregulation of CXC chemokine receptor and CD4 expression on T lymphocytes during allogeneic activation.J Immunol. 2001 Apr 15;166(8):4870-8. doi: 10.4049/jimmunol.166.8.4870. J Immunol. 2001. PMID: 11290763
-
Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45.J Biol Chem. 2003 Mar 14;278(11):9536-43. doi: 10.1074/jbc.M211803200. Epub 2003 Jan 8. J Biol Chem. 2003. PMID: 12519755
-
CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy.Immunol Lett. 2020 Jan;217:91-115. doi: 10.1016/j.imlet.2019.11.007. Epub 2019 Nov 17. Immunol Lett. 2020. PMID: 31747563 Review.
-
The good and bad faces of the CXCR4 chemokine receptor.Int J Biochem Cell Biol. 2018 Feb;95:121-131. doi: 10.1016/j.biocel.2017.12.018. Epub 2017 Dec 27. Int J Biochem Cell Biol. 2018. PMID: 29288743 Review.
Cited by
-
Impact of HIV-1 tropism on the emergence of non-AIDS events in HIV-infected patients receiving fully suppressive antiretroviral therapy.AIDS. 2016 Mar 13;30(5):731-41. doi: 10.1097/QAD.0000000000000977. AIDS. 2016. PMID: 26595543 Free PMC article.
-
Post-acute immunological and behavioral sequelae in mice after Omicron infection.bioRxiv [Preprint]. 2023 Oct 4:2023.06.05.543758. doi: 10.1101/2023.06.05.543758. bioRxiv. 2023. PMID: 37333294 Free PMC article. Preprint.
-
Increased susceptibility of CD4+ T cells from elderly individuals to HIV-1 infection and apoptosis is associated with reduced CD4 and enhanced CXCR4 and FAS surface expression levels.Retrovirology. 2015 Oct 9;12:86. doi: 10.1186/s12977-015-0213-1. Retrovirology. 2015. PMID: 26452480 Free PMC article.
-
A chronic pro-inflammatory environment contributes to the physiopathology of actinic lentigines.Sci Rep. 2024 Mar 4;14(1):5256. doi: 10.1038/s41598-024-53990-5. Sci Rep. 2024. PMID: 38438410 Free PMC article.
-
IL-2 augments the therapeutic efficacy of adoptively transferred B cells which directly kill tumor cells via the CXCR4/CXCL12 and perforin pathways.Oncotarget. 2016 Sep 13;7(37):60461-60474. doi: 10.18632/oncotarget.11124. Oncotarget. 2016. PMID: 27528023 Free PMC article.
References
-
- Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age-age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. - PubMed
-
- Bai Z, Hayasaka H, Kobayashi M, Li W, Guo Z, Jang MH, Kondo A, Choi BI, Iwakura Y, Miyasaka M. CXC chemokine ligand 12 promotes CCR7-dependent naive T cell trafficking to lymph nodes and Peyer’s patches. J Immunol. 2009;182:1287–1295. - PubMed
-
- Baum PD, Young JJ, McCune JM. Measurement of absolute T cell receptor rearrangement diversity. J Immunol Methods. 2011;368:45–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

