Socio-demographic differences in opinions about 2009 pandemic influenza A (H1N1) and seasonal influenza vaccination and disease among adults during the 2009-2010 influenza season
- PMID: 22568588
- PMCID: PMC5779826
- DOI: 10.1111/j.1750-2659.2012.00374.x
Socio-demographic differences in opinions about 2009 pandemic influenza A (H1N1) and seasonal influenza vaccination and disease among adults during the 2009-2010 influenza season
Abstract
Background: In April 2009, a novel influenza A virus emerged in the United States. By the end of July, influenza A (H1N1) 2009 monovalent (2009 H1N1) vaccine had been developed, licensed, and recommended by the Advisory Committee on Immunization Practices. Initial target groups for vaccination were identified and the first vaccine was publicly available in early October 2009.
Objective: This study examines socio-demographic differences in opinions about 2009 pandemic influenza A (H1N1) (pH1N1) and seasonal influenza disease and vaccines and the association with receipt of influenza vaccinations during the 2009-2010 influenza season. Changes in opinions over the course of the pH1N1 pandemic were also examined.
Methods: Data from the 2009 National H1N1 Flu Survey (NHFS) were analyzed. The NHFS was a CDC-sponsored telephone survey initiated in response to the 2009 pH1N1 pandemic to obtain weekly within-season estimates of vaccination coverage, opinions, and other information.
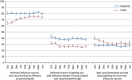
Results: Opinions about influenza vaccine and disease varied significantly by race/ethnicity, income, and education level. In multivariable logistic regression analysis, adjusted 2009 H1N1 vaccination coverage was most strongly associated with opinions about the effectiveness of the vaccine and personal risk of disease, varying from 7 to 11% among adults who believed the vaccine to have low effectiveness and themselves at low risk of influenza, to 50-53% among those who thought vaccine effectiveness to be high and themselves at high risk of influenza.
Conclusion: Improving communication about personal risk and the effectiveness of influenza vaccines may improve vaccination coverage. The findings of difference in opinions could be used to target communication.
Published 2012. This article is a US Government work and is in the public domain in the USA.
Figures

References
-
- CDC . Swine influenza A (H1N1) infection in two children – southern California, March‐April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:400–402. - PubMed
-
- CDC . The 2009 H1N1 pandemic: summary highlights, April 2009–April 2010. August 3. Available at: http://www.cdc.gov/h1n1flu/cdcresponse.htm (Accessed 22 August 2011).
-
- CDC . Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Morb Mortal Wkly Rep 2009; 58:(early release) 1–8. - PubMed
-
- CDC . Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Early Release 2009; 58:1–52. - PubMed
-
- CDC . Interim results: influenza A (H1N1) 2009 monovalent vaccination coverage – United States, October‐December 2009. MMWR Early Release 2010; 59:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

