Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine
- PMID: 22568941
- PMCID: PMC3375501
- DOI: 10.1074/jbc.C112.364620
Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine
Abstract
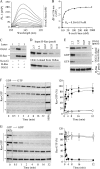
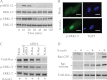
8-Oxo-7,8-dihydroguanine (8-oxoG), arguably the most abundant base lesion induced in mammalian genomes by reactive oxygen species, is repaired via the base excision repair pathway that is initiated with the excision of 8-oxoG by OGG1. Here we show that OGG1 binds the 8-oxoG base with high affinity and that the complex then interacts with canonical Ras family GTPases to catalyze replacement of GDP with GTP, thus serving as a guanine nuclear exchange factor. OGG1-mediated activation of Ras leads to phosphorylation of the mitogen-activated kinases MEK1,2/ERK1,2 and increasing downstream gene expression. These studies document for the first time that in addition to its role in repairing oxidized purines, OGG1 has an independent guanine nuclear exchange factor activity when bound to 8-oxoG.
Figures

References
-
- Mitra S., Hazra T. K., Roy R., Ikeda S., Biswas T., Lock J., Boldogh I., Izumi T. (1997) Complexities of DNA base excision repair in mammalian cells. Mol. Cells 7, 305–312 - PubMed
-
- Shibutani S., Takeshita M., Grollman A. P. (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349, 431–434 - PubMed
-
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G → T and A → C substitutions. J. Biol. Chem. 267, 166–172 - PubMed
-
- Maga G., Villani G., Crespan E., Wimmer U., Ferrari E., Bertocci B., Hübscher U. (2007) 8-Oxo-guanine bypass by human DNA polymerases in the presence of auxiliary proteins. Nature 447, 606–608 - PubMed
-
- Haracska L., Yu S. L., Johnson R. E., Prakash L., Prakash S. (2000) Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nat. Genet. 25, 458–461 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

