Blocking of bradykinin receptor B1 protects from focal closed head injury in mice by reducing axonal damage and astroglia activation
- PMID: 22569191
- PMCID: PMC3434625
- DOI: 10.1038/jcbfm.2012.62
Blocking of bradykinin receptor B1 protects from focal closed head injury in mice by reducing axonal damage and astroglia activation
Abstract
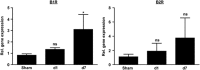
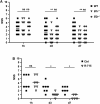
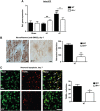
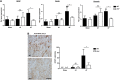
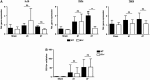
The two bradykinin receptors B1R and B2R are central components of the kallikrein-kinin system with different expression kinetics and binding characteristics. Activation of these receptors by kinins triggers inflammatory responses in the target organ and in most situations enhances tissue damage. We could recently show that blocking of B1R, but not B2R, protects from cortical cryolesion by reducing inflammation and edema formation. In the present study, we investigated the role of B1R and B2R in a closed head model of focal traumatic brain injury (TBI; weight drop). Increased expression of B1R in the injured hemispheres of wild-type mice was restricted to the later stages after brain trauma, i.e. day 7 (P<0.05), whereas no significant induction could be observed for the B2R (P>0.05). Mice lacking the B1R, but not the B2R, showed less functional deficits on day 3 (P<0.001) and day 7 (P<0.001) compared with controls. Pharmacological blocking of B1R in wild-type mice had similar effects. Reduced axonal injury and astroglia activation could be identified as underlying mechanisms, while inhibition of B1R had only little influence on the local inflammatory response in this model. Inhibition of B1R may become a novel strategy to counteract trauma-induced neurodegeneration.
Figures
References
-
- Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, Renne T, Kleinschnitz C. Blockade of bradykinin receptor B1 but not bradykinin receptor B2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40:285–293. - PubMed
-
- Buki A, Povlishock JT.2006All roads lead to disconnection?—Traumatic axonal injury revisited Acta Neurochir (Wien) 148181–193.discussion 93–4 - PubMed
-
- Camargo AC, Shapanka R, Greene LJ. Preparation, assay, and partial characterization of a neutral endopeptidase from rabbit brain. Biochemistry. 1973;12:1838–1844. - PubMed
-
- Cholewinski AJ, Stevens G, McDermott AM, Wilkin GP. Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem. 1991;57:1456–1458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

