Severity score system for progressive myelopathy: development and validation of a new clinical scale
- PMID: 22570090
- PMCID: PMC3854272
- DOI: 10.1590/s0100-879x2012007500072
Severity score system for progressive myelopathy: development and validation of a new clinical scale
Abstract
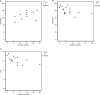
Progressive myelopathies can be secondary to inborn errors of metabolism (IEM) such as mucopolysaccharidosis, mucolipidosis, and adrenomyeloneuropathy. The available scale, Japanese Orthopaedic Association (JOA) score, was validated only for degenerative vertebral diseases. Our objective is to propose and validate a new scale addressing progressive myelopathies and to present validating data for JOA in these diseases. A new scale, Severity Score System for Progressive Myelopathy (SSPROM), covering motor disability, sphincter dysfunction, spasticity, and sensory losses. Inter- and intra-rater reliabilities were measured. External validation was tested by applying JOA, the Expanded Disability Status Scale (EDSS), the Barthel index, and the Osame Motor Disability Score. Thirty-eight patients, 17 with adrenomyeloneuropathy, 3 with mucopolysaccharidosis I, 3 with mucopolysaccharidosis IV, 2 with mucopolysaccharidosis VI, 2 with mucolipidosis, and 11 with human T-cell lymphotropic virus type-1 (HTLV-1)-associated myelopathy participated in the study. The mean ± SD SSPROM and JOA scores were 74.6 ± 11.4 and 12.4 ± 2.3, respectively. Construct validity for SSPROM (JOA: r = 0.84, P < 0.0001; EDSS: r = -0.83, P < 0.0001; Barthel: r = 0.56, P < 0.002; Osame: r = -0.94, P < 0.0001) and reliability (intra-rater: r = 0.83, P < 0.0001; inter-rater: r = 0.94, P < 0.0001) were demonstrated. The metric properties of JOA were similar to those found in SSPROM. Several clinimetric requirements were met for both SSPROM and JOA scales. Since SSPROM has a wider range, it should be useful for follow-up studies on IEM myelopathies.
Figures



References
-
- Byrne TN, Waxman SG. Neurology in clinical practice. Philadelphia: Butterworth Heinemann; 2004. Paraplegia and spinal cord syndromes; pp. 351–366. (Editors)
-
- Muenzer J. The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J Pediatr. 2004;144:S27–S34. - PubMed
-
- Kachur E, Del Maestro R. Mucopolysaccharidoses and spinal cord compression: case report and review of the literature with implications of bone marrow transplantation. Neurosurgery. 2000;47:223–228. - PubMed
-
- Carl A, Waldman J, Malone A, Blair B. Atlantoaxial instability and myelopathy in mucolipidosis. Spine. 1991;16:215–217. - PubMed
-
- Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain. 1997;120 (Part 8):1485–1508. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

