Translational evidence that impaired autophagy contributes to arterial ageing
- PMID: 22570377
- PMCID: PMC3459044
- DOI: 10.1113/jphysiol.2012.229690
Translational evidence that impaired autophagy contributes to arterial ageing
Abstract
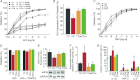
Ageing causes arterial endothelial dysfunction that increases the risk of cardiovascular diseases (CVD), but the underlying mechanisms are incompletely understood. The aim of the present study was to determine the role of autophagy, the cellular process of recycling damaged biomolecules, in endothelial dysfunction with ageing. In older humans, expression of autophagy markers in arterial endothelial cells was impaired by ∼50% (P <0.05) and was associated with an ∼30% (P <0.05) reduction in arterial endothelium-dependent dilatation (EDD). Similarly, in C57BL/6 control mice ageing was associated with an ∼40% decrease (P <0.05) in arterial markers of autophagy and an ∼25% reduction (P <0.05) in EDD. In both humans and mice, impaired EDD was mediated by reduced nitric oxide (NO) bioavailability and was associated with increased oxidative stress and inflammation (P <0.05). In old mice, treatment with the autophagy-enhancing agent trehalose restored expression of autophagy markers, rescued NO-mediated EDD by reducing oxidative stress, and normalized inflammatory cytokine expression. In cultured endothelial cells, inhibition of autophagy increased oxidative stress and reduced NO production, whereas trehalose enhanced NO production via an autophagy-dependent mechanism. These results provide the first evidence that autophagy is impaired with ageing in vascular tissues. Our findings also suggest that autophagy preserves arterial endothelial function by reducing oxidative stress and inflammation and increasing NO bioavailability. Autophagy-enhancing strategies may therefore have therapeutic efficacy for ameliorating age-associated arterial dysfunction and preventing CVD.
Figures
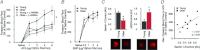
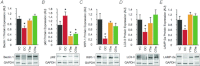



References
-
- Arai C, Arai N, Mizote A, Kohno K, Iwaki K, Hanaya T, Arai S, Ushio S, Fukuda S. Trehalose prevents adipocyte hypertrophy and mitigates insulin resistance. Nutr Res. 2010;30:840–848. - PubMed
-
- Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. - PubMed
-
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–2453. - PubMed
-
- Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2001;4:91–102. - PubMed
-
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

