Mycobacterium tuberculosis RbpA protein is a new type of transcriptional activator that stabilizes the σ A-containing RNA polymerase holoenzyme
- PMID: 22570422
- PMCID: PMC3413145
- DOI: 10.1093/nar/gks346
Mycobacterium tuberculosis RbpA protein is a new type of transcriptional activator that stabilizes the σ A-containing RNA polymerase holoenzyme
Abstract
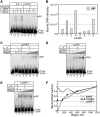
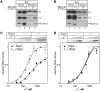
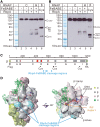
RbpA is an RNA polymerase (RNAP)-binding protein whose presence increases the tolerance levels of Mycobacteria to the first-line anti-tuberculosis drug rifampicin by an unknown mechanism. Here, we show that the role of Mycobacterium tuberculosis RbpA in resistance is indirect because it does not affect the sensitivity of RNAP to rifampicin while it stimulates transcription controlled by the housekeeping σ(A)-factor. The transcription regulated by the stress-related σ(F) was not affected by RbpA. The binding site of RbpA maps to the RNAP β subunit Sandwich-Barrel Hybrid Motif, which has not previously been described as an activator target and does not overlap the rifampicin binding site. Our data suggest that RbpA modifies the structure of the core RNAP, increases its affinity for σ(A) and facilitates the assembly of the transcriptionally competent promoter complexes. We propose that RbpA is an essential partner which advantages σ(A) competitiveness for core RNAP binding with respect to the alternative σ factors. The RbpA-driven stimulation of the housekeeping gene expression may help Mycobacteria to tolerate high rifampicin levels and to adapt to the stress conditions during infection.
Figures


Similar articles
-
The σ Subunit-Remodeling Factors: An Emerging Paradigms of Transcription Regulation.Front Microbiol. 2020 Jul 29;11:1798. doi: 10.3389/fmicb.2020.01798. eCollection 2020. Front Microbiol. 2020. PMID: 32849409 Free PMC article. Review.
-
Mycobacterium RbpA cooperates with the stress-response σB subunit of RNA polymerase in promoter DNA unwinding.Nucleic Acids Res. 2014;42(16):10399-408. doi: 10.1093/nar/gku742. Epub 2014 Aug 13. Nucleic Acids Res. 2014. PMID: 25122744 Free PMC article.
-
The RNA polymerase-binding protein RbpA confers basal levels of rifampicin resistance on Streptomyces coelicolor.Mol Microbiol. 2006 May;60(3):687-96. doi: 10.1111/j.1365-2958.2006.05116.x. Mol Microbiol. 2006. PMID: 16629670
-
Mycobacterium tuberculosis RNA polymerase-binding protein A (RbpA) and its interactions with sigma factors.J Biol Chem. 2013 May 17;288(20):14438-14450. doi: 10.1074/jbc.M113.459883. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548911 Free PMC article.
-
Mechanisms for activating bacterial RNA polymerase.FEMS Microbiol Rev. 2010 Sep;34(5):611-27. doi: 10.1111/j.1574-6976.2010.00239.x. Epub 2010 Jun 7. FEMS Microbiol Rev. 2010. PMID: 20629756 Review.
Cited by
-
MoaB2, a newly identified transcription factor, binds to σA in Mycobacterium smegmatis.J Bacteriol. 2024 Dec 19;206(12):e0006624. doi: 10.1128/jb.00066-24. Epub 2024 Nov 5. J Bacteriol. 2024. PMID: 39499088 Free PMC article.
-
Genome-Based Insights into the Production of Carotenoids by Antarctic Bacteria, Planococcus sp. ANT_H30 and Rhodococcus sp. ANT_H53B.Molecules. 2020 Sep 23;25(19):4357. doi: 10.3390/molecules25194357. Molecules. 2020. PMID: 32977394 Free PMC article.
-
The σ Subunit-Remodeling Factors: An Emerging Paradigms of Transcription Regulation.Front Microbiol. 2020 Jul 29;11:1798. doi: 10.3389/fmicb.2020.01798. eCollection 2020. Front Microbiol. 2020. PMID: 32849409 Free PMC article. Review.
-
High-throughput, fluorescent-aptamer-based measurements of steady-state transcription rates for the Mycobacterium tuberculosis RNA polymerase.Nucleic Acids Res. 2023 Oct 27;51(19):e99. doi: 10.1093/nar/gkad761. Nucleic Acids Res. 2023. PMID: 37739412 Free PMC article.
-
Structural, functional, and genetic analyses of the actinobacterial transcription factor RbpA.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7171-6. doi: 10.1073/pnas.1504942112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26040003 Free PMC article.
References
-
- WHO. (2011) Global tuberculosis control. Available online at http://www.who.int/entity/tb/publications/global_report/2011/gtbr11_full....
-
- Tupin A, Gualtieri M, Roquet-Banères F, Morichaud Z, Brodolin K, Leonetti J. Resistance to rifampicin: at the crossroads between ecological, genomic and medical concerns. Int. J. Antimicrob. Agents. 2010;35:519–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

