The lipopolysaccharide from Capnocytophaga canimorsus reveals an unexpected role of the core-oligosaccharide in MD-2 binding
- PMID: 22570611
- PMCID: PMC3342949
- DOI: 10.1371/journal.ppat.1002667
The lipopolysaccharide from Capnocytophaga canimorsus reveals an unexpected role of the core-oligosaccharide in MD-2 binding
Abstract
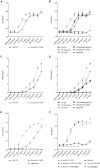
Capnocytophaga canimorsus is a usual member of dog's mouths flora that causes rare but dramatic human infections after dog bites. We determined the structure of C. canimorsus lipid A. The main features are that it is penta-acylated and composed of a "hybrid backbone" lacking the 4' phosphate and having a 1 phosphoethanolamine (P-Etn) at 2-amino-2-deoxy-d-glucose (GlcN). C. canimorsus LPS was 100 fold less endotoxic than Escherichia coli LPS. Surprisingly, C. canimorsus lipid A was 20,000 fold less endotoxic than the C. canimorsus lipid A-core. This represents the first example in which the core-oligosaccharide dramatically increases endotoxicity of a low endotoxic lipid A. The binding to human myeloid differentiation factor 2 (MD-2) was dramatically increased upon presence of the LPS core on the lipid A, explaining the difference in endotoxicity. Interaction of MD-2, cluster of differentiation antigen 14 (CD14) or LPS-binding protein (LBP) with the negative charge in the 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) of the core might be needed to form the MD-2 - lipid A complex in case the 4' phosphate is not present.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

