A novel human polycomb binding site acts as a functional polycomb response element in Drosophila
- PMID: 22570707
- PMCID: PMC3343078
- DOI: 10.1371/journal.pone.0036365
A novel human polycomb binding site acts as a functional polycomb response element in Drosophila
Abstract
Polycomb group (PcG) proteins are key chromatin regulators implicated in multiple processes including embryonic development, tissue homeostasis, genomic imprinting, X-chromosome inactivation, and germ cell differentiation. The PcG proteins recognize target genomic loci through cis DNA sequences known as Polycomb Response Elements (PREs), which are well characterized in Drosophila. However, mammalian PREs have been elusive until two groups reported putative mammalian PREs recently. Consistent with the existence of mammalian PREs, here we report the identification and characterization of a potential PRE from human T cells. The putative human PRE has enriched binding of PcG proteins, and such binding is dependent on a key PcG component SUZ12. We demonstrate that the putative human PRE carries both genetic and molecular features of Drosophila PRE in transgenic flies, implying that not only the trans PcG proteins but also certain features of the cis PREs are conserved between mammals and Drosophila.
Conflict of interest statement
Figures




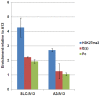
References
-
- Simon JA. Polycomb group proteins. Curr Biol. 2003;13:R79–80. - PubMed
-
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. - PubMed
-
- Levine SS, King IF, Kingston RE. Division of labor in polycomb group repression. Trends Biochem Sci. 2004;29:478–485. - PubMed
-
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. - PubMed
-
- Orlando V. Polycomb, epigenomes, and control of cell identity. Cell. 2003;112:599–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

