Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes
- PMID: 22570710
- PMCID: PMC3343052
- DOI: 10.1371/journal.pone.0036402
Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes
Abstract
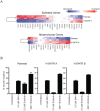
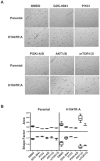
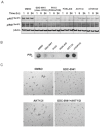
The PTEN/PI3K pathway is commonly mutated in cancer and therefore represents an attractive target for therapeutic intervention. To investigate the primary phenotypes mediated by increased pathway signaling in a clean, patient-relevant context, an activating PIK3CA mutation (H1047R) was knocked-in to an endogenous allele of the MCF10A non-tumorigenic human breast epithelial cell line. Introduction of an endogenously mutated PIK3CA allele resulted in a marked epithelial-mesenchymal transition (EMT) and invasive phenotype, compared to isogenic wild-type cells. The invasive phenotype was linked to enhanced PIP(3) production via a S6K-IRS positive feedback mechanism. Moreover, potent and selective inhibitors of PI3K were highly effective in reversing this phenotype, which is optimally revealed in 3-dimensional cell culture. In contrast, inhibition of Akt or mTOR exacerbated the invasive phenotype. Our results suggest that invasion is a core phenotype mediated by increased PTEN/PI3K pathway activity and that therapeutic agents targeting different nodes of the PI3K pathway may have dramatic differences in their ability to reverse or promote cancer metastasis.
Conflict of interest statement
Figures



References
-
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. - PubMed
-
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. - PubMed
-
- Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clin Cancer Res. 2010;16:4325–4330. - PubMed
-
- Haruta T, Uno T, Kawahara J, Takano A, Egawa K, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

