Low-dose, ionizing radiation and age-related changes in skeletal microarchitecture
- PMID: 22570786
- PMCID: PMC3337602
- DOI: 10.1155/2012/481983
Low-dose, ionizing radiation and age-related changes in skeletal microarchitecture
Abstract
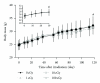
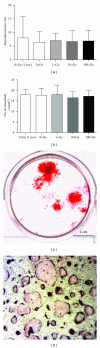
Osteoporosis can profoundly affect the aged as a consequence of progressive bone loss; high-dose ionizing radiation can cause similar changes, although less is known about lower doses (≤100 cGy). We hypothesized that exposure to relatively low doses of gamma radiation accelerates structural changes characteristic of skeletal aging. Mice (C57BL/6J-10 wk old, male) were irradiated (total body; 0-sham, 1, 10 or 100 cGy (137)Cs) and tissues harvested on the day of irradiation, 1 or 4 months later. Microcomputed tomography was used to quantify microarchitecture of high turnover, cancellous bone. Irradiation at 100 cGy caused transient microarchitectural changes over one month that were only evident at longer times in controls (4 months). Ex vivo bone cell differentiation from the marrow was unaffected by gamma radiation. In conclusion, acute ionizing gamma irradiation at 100 cGy (but not at 1 cGy or 10 cGy) exacerbated microarchitectural changes normally found during progressive, postpubertal aging prior to the onset of age-related osteoporosis.
Figures

References
-
- Seeman E. Invited review: pathogenesis of osteoporosis. Journal of Applied Physiology. 2003;95(5):2142–2151. - PubMed
-
- Baxter-Jones ADG, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. Journal of Bone and Mineral Research. 2011;26(8):1729–1739. - PubMed
-
- Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. Journal of Bone and Mineral Research. 2002;17(6):1044–1050. - PubMed
-
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. Journal of Bone and Mineral Research. 2007;22(8):1197–1207. - PubMed
-
- Friedenstein AJ. Bone marrow osteogenic stem cells. In: Cohn DV, Glorieux FH, Martin TJ, editors. Calcium Regulation and Bone Metabolism. Elsevier Science Publishers; 1990. pp. 353–360.

