Studies toward the synthesis of the epoxykinamycin FL-120B': discovery of a decarbonylative photocyclization
- PMID: 22571279
- PMCID: PMC3433630
- DOI: 10.1021/ol3010563
Studies toward the synthesis of the epoxykinamycin FL-120B': discovery of a decarbonylative photocyclization
Abstract
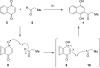
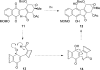
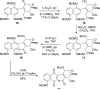
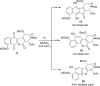
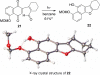
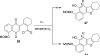
Photo-Friedel-Crafts acylation of a naphthoquinone was attempted in an effort to access a diazobenzofluorenone en route to the epoxykinamycin natural product FL-120B'. Photoirradiation of the naphthoquinone substrate which resulted in the unexpected formation of a tetracyclic naphthofuran via a decarbonylative photocyclization process is described.
Figures

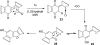

References
-
-
For a recent review including synthetic and mechanism-of-action studies of diazobenzofluorene natural products, see: Herzon SB, Woo CM. Nat. Prod. Rep. 2012;29:87–118.
-
-
-
For a recent example of serendipitous reaction discovery in studies toward diazobenzofluorene natural products, see: Baranczak A, Sulikowski GA. Org. Lett. 2012;14:1027–1029.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

