Protein lysine acylation and cysteine succination by intermediates of energy metabolism
- PMID: 22571489
- PMCID: PMC3376250
- DOI: 10.1021/cb3001793
Protein lysine acylation and cysteine succination by intermediates of energy metabolism
Abstract
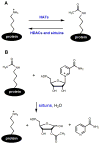
In the past few years, several new protein post-translational modifications that use intermediates in metabolism have been discovered. These include various acyl lysine modifications (formylation, propionylation, butyrylation, crotonylation, malonylation, succinylation, myristoylation) and cysteine succination. Here, we review the discovery and the current understanding of these modifications. Several of these modifications are regulated by the deacylases, sirtuins, which use nicotinamide adenine dinucleotide (NAD), an important metabolic small molecule. Interestingly, several of these modifications in turn regulate the activity of metabolic enzymes. These new modifications reveal interesting connections between metabolism and protein post-translational modifications and raise many questions for future investigations.
Figures







References
-
- Walsh CT. Posttranslational Modification of Proteins: Expanding Nature’s Inventory. Roberts and Company Publishers; Englewood, Colorado: 2005.
-
- Frizzell N, Lima M, Baynes JW. Succination of proteins in diabetes. Free Radic Res. 2011;45:101–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

