Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells
- PMID: 22571869
- PMCID: PMC3516844
- DOI: 10.1158/1541-7786.MCR-11-0616
Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells
Abstract
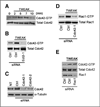
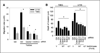
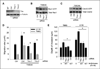
Malignant glioblastomas are characterized by their ability to infiltrate into normal brain. We previously reported that binding of the multifunctional cytokine TNF-like weak inducer of apoptosis (TWEAK) to its receptor fibroblast growth factor-inducible 14 (Fn14) induces glioblastoma cell invasion via Rac1 activation. Here, we show that Cdc42 plays an essential role in Fn14-mediated activation of Rac1. TWEAK-treated glioma cells display an increased activation of Cdc42, and depletion of Cdc42 using siRNA abolishes TWEAK-induced Rac1 activation and abrogates glioma cell migration and invasion. In contrast, Rac1 depletion does not affect Cdc42 activation by Fn14, showing that Cdc42 mediates TWEAK-stimulated Rac1 activation. Furthermore, we identified two guanine nucleotide exchange factors (GEF), Ect2 and Trio, involved in TWEAK-induced activation of Cdc42 and Rac1, respectively. Depletion of Ect2 abrogates both TWEAK-induced Cdc42 and Rac1 activation, as well as subsequent TWEAK-Fn14-directed glioma cell migration and invasion. In contrast, Trio depletion inhibits TWEAK-induced Rac1 activation but not TWEAK-induced Cdc42 activation. Finally, inappropriate expression of Fn14 or Ect2 in mouse astrocytes in vivo using an RCAS vector system for glial-specific gene transfer in G-tva transgenic mice induces astrocyte migration within the brain, corroborating the in vitro importance of the TWEAK-Fn14 signaling cascade in glioblastoma invasion. Our results suggest that the TWEAK-Fn14 signaling axis stimulates glioma cell migration and invasion through two GEF-GTPase signaling units, Ect2-Cdc42 and Trio-Rac1. Components of the Fn14-Rho GEF-Rho GTPase signaling pathway present innovative drug targets for glioma therapy.
Mol Cancer Res; 10(7); 958-68. ©2012 AACR.
Conflict of interest statement
No potential conflicts of interests were disclosed.
Figures




References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. - PubMed
-
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

