Complex autonomous firing patterns of striatal low-threshold spike interneurons
- PMID: 22572945
- PMCID: PMC3424086
- DOI: 10.1152/jn.00283.2012
Complex autonomous firing patterns of striatal low-threshold spike interneurons
Abstract
During sensorimotor learning, tonically active neurons (TANs) in the striatum acquire bursts and pauses in their firing based on the salience of the stimulus. Striatal cholinergic interneurons display tonic intrinsic firing, even in the absence of synaptic input, that resembles TAN activity seen in vivo. However, whether there are other striatal neurons among the group identified as TANs is unknown. We used transgenic mice expressing green fluorescent protein under control of neuronal nitric oxide synthase or neuropeptide-Y promoters to aid in identifying low-threshold spike (LTS) interneurons in brain slices. We found that these neurons exhibit autonomous firing consisting of spontaneous transitions between regular, irregular, and burst firing, similar to cholinergic interneurons. As in cholinergic interneurons, these firing patterns arise from interactions between multiple intrinsic oscillatory mechanisms, but the mechanisms responsible differ. Both neurons maintain tonic firing because of persistent sodium currents, but the mechanisms of the subthreshold oscillations responsible for irregular firing are different. In LTS interneurons they rely on depolarization-activated noninactivating calcium currents, whereas those in cholinergic interneurons arise from a hyperpolarization-activated potassium conductance. Sustained membrane hyperpolarizations induce a bursting pattern in LTS interneurons, probably by recruiting a low-threshold, inactivating calcium conductance and by moving the membrane potential out of the activation range of the oscillatory mechanisms responsible for single spiking, in contrast to the bursting driven by noninactivating currents in cholinergic interneurons. The complex intrinsic firing patterns of LTS interneurons may subserve differential release of classic and peptide neurotransmitters as well as nitric oxide.
Figures
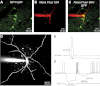
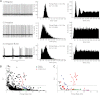
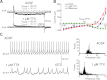

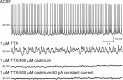
References
-
- Adewale AS, Macarthur H, Westfall TC. Neuropeptide Y-induced enhancement of the evoked release of newly synthesized dopamine in rat striatum: mediation by Y2 receptors. Neuropharmacology 52: 1396–1402, 2007 - PubMed
-
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature 344: 240–242, 1990 - PubMed
-
- Albe-Fessard D, Rocha-Miranda C, Oswaldo-Cruz E. Activités évoquées dans le noyau caudé du chat en réponse à des types divers d'afférences (in French). Electroenceph Clin Neurophysiol 12: 649–661, 1960 - PubMed
-
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol 53: 1417–1430, 1985 - PubMed
-
- Anderson ME. Discharge patterns of basal ganglia neurons during active maintenance of postural stability and adjustment to chair tilt. Brain Res 143: 325–338, 1977 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

