Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice
- PMID: 22572998
- PMCID: PMC3431429
- DOI: 10.1095/biolreprod.111.096511
Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice
Abstract
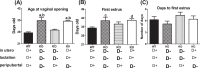
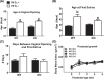
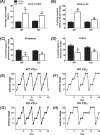
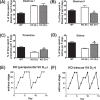
The mechanism(s) by which vitamin D(3) regulates female reproduction is minimally understood. We tested the hypothesis that peripubertal vitamin D(3) deficiency disrupts hypothalamic-pituitary-ovarian physiology. To test this hypothesis, we used wild-type mice and Cyp27b1 (the rate-limiting enzyme in the synthesis of 1,25-dihydroxyvitamin D(3)) null mice to study the effect of vitamin D(3) deficiency on puberty and reproductive physiology. At the time of weaning, mice were randomized to a vitamin D(3)-replete or -deficient diet supplemented with calcium. We assessed the age of vaginal opening and first estrus (puberty markers), gonadotropin levels, ovarian histology, ovarian responsiveness to exogenous gonadotropins, and estrous cyclicity. Peripubertal vitamin D(3) deficiency significantly delayed vaginal opening without affecting the number of GnRH-immunopositive neurons or estradiol-negative feedback on gonadotropin levels during diestrus. Young adult females maintained on a vitamin D(3)-deficient diet after puberty had arrested follicular development and prolonged estrous cycles characterized by extended periods of diestrus. Ovaries of vitamin D(3)-deficient Cyp27b1 null mice responded to exogenous gonadotropins and deposited significantly more oocytes into the oviducts than mice maintained on a vitamin D(3)-replete diet. Estrous cycles were restored when vitamin D(3)-deficient Cyp27b1 null young adult females were transferred to a vitamin D(3)-replete diet. This study is the first to demonstrate that peripubertal vitamin D(3) sufficiency is important for an appropriately timed pubertal transition and maintenance of normal female reproductive physiology. These data suggest vitamin D(3) is a key regulator of neuroendocrine and ovarian physiology.
Figures



References
-
- Christakos S, Barletta F, Huening M, Dhawan P, Liu Y, Porta A, Peng X. Vitamin D target proteins: function and regulation. J Cell Biochem 2003; 88 (2): 238 244 - PubMed
-
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem 2003; 88 (4): 695 705 - PubMed
-
- Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, Feron F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 2007; 103 (3–5): 538 545 - PubMed
-
- Hakim I, Bar-Shavit Z. Modulation of TNF-alpha expression in bone marrow macrophages: involvement of vitamin D response element. J Cell Biochem 2003; 88 (5): 986 998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

