Differential distribution of diacylglycerol lipase-alpha and N-acylphosphatidylethanolamine-specific phospholipase d immunoreactivity in the superficial spinal dorsal horn of rats
- PMID: 22573306
- PMCID: PMC3703832
- DOI: 10.1002/glia.22351
Differential distribution of diacylglycerol lipase-alpha and N-acylphosphatidylethanolamine-specific phospholipase d immunoreactivity in the superficial spinal dorsal horn of rats
Abstract
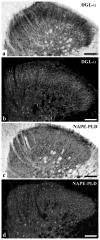
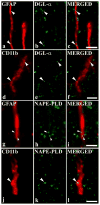
It is generally accepted that the endocannabinoid system plays important roles in spinal pain processing. Although it is documented that cannabinoid-1 receptors are strongly expressed in the superficial spinal dorsal horn, the cellular distribution of enzymes that can synthesize endocannabinoid ligands is less well studied. Thus, using immunocytochemical methods at the light and electron microscopic levels, we investigated the distribution of diacylglycerol lipase-alpha (DGL-α) and N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD), enzymes synthesizing the endocannabinoid ligands, 2-arachidonoylglycerol (2-AG) and anandamide, respectively. Positive labeling was revealed only occasionally in axon terminals, but dendrites displayed strong immunoreactivity for both enzymes. However, the dendritic localization of DGL-α and NAPE-PLD showed a remarkably different distribution. DGL-α immunolabeling in dentrites was always revealed at membrane compartments in close vicinity to synapses. In contrast to this, dendritic NAPE-PLD labeling was never observed in association with synaptic contacts. In addition to dendrites, a substantial proportion of astrocytic (immunoreactive for GFAP) and microglial (immunoreactive for CD11b) profiles were also immunolabeled for both DGL-α and NAPE-PLD. Glial processes immunostained for DGL-α were frequently found near to synapses in which the postsynaptic dendrite was immunoreactive for DGL-α, whereas NAPE-PLD immunoreactivity on glial profiles at the vicinity of synapses was only occasionally observed. Our results suggest that both neurons and glial cells can synthesize and release 2-AG and anandamide in the superficial spinal dorsal horn. The 2-AG can primarily be released by postsynaptic dendrites and glial processes adjacent to synapses, whereas anandamide can predominantly be released from nonsynaptic dendritic and glial compartments.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




Similar articles
-
Ultrastructural evidence for synaptic contacts between cortical noradrenergic afferents and endocannabinoid-synthesizing post-synaptic neurons.Neuroscience. 2015 Sep 10;303:323-37. doi: 10.1016/j.neuroscience.2015.07.009. Epub 2015 Jul 8. Neuroscience. 2015. PMID: 26162236 Free PMC article.
-
Neuronal and glial localization of the cannabinoid-1 receptor in the superficial spinal dorsal horn of the rodent spinal cord.Eur J Neurosci. 2009 Jul;30(2):251-62. doi: 10.1111/j.1460-9568.2009.06816.x. Epub 2009 Jul 15. Eur J Neurosci. 2009. PMID: 19614976
-
Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia.Eur J Neurosci. 2009 May;29(10):1964-78. doi: 10.1111/j.1460-9568.2009.06751.x. Epub 2009 May 9. Eur J Neurosci. 2009. PMID: 19453631 Free PMC article.
-
Biology of endocannabinoid synthesis system.Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):112-9. doi: 10.1016/j.prostaglandins.2008.12.002. Epub 2008 Dec 14. Prostaglandins Other Lipid Mediat. 2009. PMID: 19126434 Review.
-
Biosynthetic pathways of the endocannabinoid anandamide.Chem Biodivers. 2007 Aug;4(8):1842-57. doi: 10.1002/cbdv.200790155. Chem Biodivers. 2007. PMID: 17712822 Review.
Cited by
-
Promising cannabinoid-based therapies for Parkinson's disease: motor symptoms to neuroprotection.Mol Neurodegener. 2015 Apr 8;10:17. doi: 10.1186/s13024-015-0012-0. Mol Neurodegener. 2015. PMID: 25888232 Free PMC article. Review.
-
Inhibition of synaptic transmission by anandamide precursor 20:4-NAPE is mediated by TRPV1 receptors under inflammatory conditions.Front Mol Neurosci. 2023 Jun 22;16:1188503. doi: 10.3389/fnmol.2023.1188503. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37426071 Free PMC article.
-
The Endocannabinoid System and Invertebrate Neurodevelopment and Regeneration.Int J Mol Sci. 2021 Feb 20;22(4):2103. doi: 10.3390/ijms22042103. Int J Mol Sci. 2021. PMID: 33672634 Free PMC article. Review.
-
The Development of Cannabinoids as Therapeutic Agents in the United States.Pharmacol Rev. 2024 Aug 15;76(5):915-955. doi: 10.1124/pharmrev.123.001121. Pharmacol Rev. 2024. PMID: 38849155 Free PMC article. Review.
-
Reactive spinal glia convert 2-AG to prostaglandins to drive aberrant astroglial calcium signaling.Front Cell Neurosci. 2024 May 9;18:1382465. doi: 10.3389/fncel.2024.1382465. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38784707 Free PMC article.
References
-
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. - PubMed
-
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between neurons and glia. Annu Rev Physiol. 2001;63:795–813. - PubMed
-
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport. 2000;11:1231–1235. - PubMed
-
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gagadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. - PMC - PubMed
-
- Bisogno T, Melck D, De Petrocellis L, Di Marzo V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem. 1999;72:2113–2119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

