Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration
- PMID: 22573321
- PMCID: PMC3408142
- DOI: 10.1074/jbc.M112.349126
Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration
Abstract
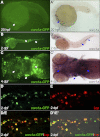
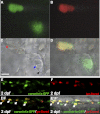
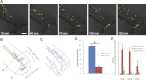
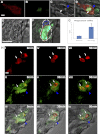
Macrophages and neutrophils are the pivotal immune phagocytes that enter the wound after tissue injury to remove the cell debris and invaded microorganisms, which presumably facilitate the regrowth of injured tissues. Taking advantage of the regeneration abilities of zebrafish and the newly generated leukocyte-specific zebrafish lines with labeling of both leukocyte lineages, we assessed the behaviors and functions of neutrophils and macrophages during tail fin regeneration. Live imaging showed that within 6 hours post amputation, the inflammatory stage, neutrophils were the primary cells scavenging apoptotic bodies and small cell debris, although they had limited phagocytic capacity and quickly underwent apoptosis. From 6 hours post amputation on, the resolution and regeneration stage, macrophages became the dominant scavengers, efficiently resolving inflammation and facilitating tissue remodeling and regrowth. Ablation of macrophages but not neutrophils severely impaired the inflammatory resolution and tissue regeneration, resulting in the formation of large vacuoles in the regenerated fins. In contrast, removal of neutrophils slightly accelerates the regrowth of injured fin. Our study documents the differing behaviors and functions of macrophages and neutrophils during tissue regeneration.
Figures
References
-
- Poss K. D., Keating M. T., Nechiporuk A. (2003) Tales of regeneration in zebrafish. Dev. Dyn. 226, 202–210 - PubMed
-
- Medzhitov R. (2007) Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 - PubMed
-
- Martin P., Leibovich S. J. (2005) Inflammatory cells during wound repair. The good, the bad, and the ugly. Trends Cell Biol. 15, 599–607 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

