Matrix metalloprotease-1a promotes tumorigenesis and metastasis
- PMID: 22573325
- PMCID: PMC3397859
- DOI: 10.1074/jbc.M112.356303
Matrix metalloprotease-1a promotes tumorigenesis and metastasis
Abstract
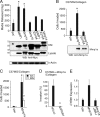
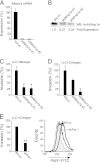
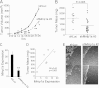
Matrix metalloprotease-1 (MMP1), a collagenase and activator of the G protein-coupled protease activated receptor-1 (PAR1), is an emerging new target implicated in oncogenesis and metastasis in diverse cancers. However, the functional mouse homologue of MMP1 in cancer models has not yet been clearly defined. We report here that Mmp1a is a functional MMP1 homologue that promotes invasion and metastatic progression of mouse lung cancer and melanoma. LLC1 (Lewis lung carcinoma) and primary mouse melanoma cells harboring active BRAF express high levels of endogenous Mmp1a, which is required for invasion through collagen. Silencing of either Mmp1a or PAR1 suppressed invasive stellate growth of lung cancer cells in three-dimensional matrices. Conversely, ectopic expression of Mmp1a conferred an invasive phenotype in epithelial cells that do not express endogenous Mmp1a. Consistent with Mmp1a acting as a PAR1 agonist in an autocrine loop, inhibition or silencing of PAR1 resulted in a loss of the Mmp1a-driven invasive phenotype. Knockdown of Mmp1a on tumor cells resulted in significantly decreased tumorigenesis, invasion, and metastasis in xenograft models. Together, these data demonstrate that cancer cell-derived Mmp1a acts as a robust functional homologue of MMP1 by conferring protumorigenic and metastatic behavior to cells.
Figures



References
-
- Ugalde A. P., Ordóñez G. R., Quirós P. M., Puente X. S., López-Otín C. (2010) Metalloproteases and the degradome. Methods Mol. Biol. 622, 3–29 - PubMed
-
- Rodríguez D., Morrison C. J., Overall C. M. (2010) Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta 1803, 39–54 - PubMed
-
- Fanjul-Fernández M., Folgueras A. R., Cabrera S., López-Otín C. (2010) Matrix metalloproteinases: Evolution, gene regulation, and functional analysis in mouse models. Biochim. Biophys. Acta 1803, 3–19 - PubMed
-
- Minond D., Lauer-Fields J. L., Cudic M., Overall C. M., Pei D., Brew K., Visse R., Nagase H., Fields G. B. (2006) The roles of substrate thermal stability and P2 and P1′ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J. Biol. Chem. 281, 38302–38313 - PubMed
-
- Brinckerhoff C. E., Rutter J. L., Benbow U. (2000) Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 6, 4823–4830 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

