Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure
- PMID: 22573329
- PMCID: PMC3391150
- DOI: 10.1074/jbc.M111.335935
Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure
Abstract
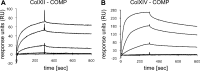
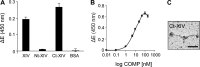
The tensile and scaffolding properties of skin rely on the complex extracellular matrix (ECM) that surrounds cells, vasculature, nerves, and adnexus structures and supports the epidermis. In the skin, collagen I fibrils are the major structural component of the dermal ECM, decorated by proteoglycans and by fibril-associated collagens with interrupted triple helices such as collagens XII and XIV. Here we show that the cartilage oligomeric matrix protein (COMP), an abundant component of cartilage ECM, is expressed in healthy human skin. COMP expression is detected in the dermal compartment of skin and in cultured fibroblasts, whereas epidermis and HaCaT cells are negative. In addition to binding collagen I, COMP binds to collagens XII and XIV via their C-terminal collagenous domains. All three proteins codistribute in a characteristic narrow zone in the superficial papillary dermis of healthy human skin. Ultrastructural analysis by immunogold labeling confirmed colocalization and further revealed the presence of COMP along with collagens XII and XIV in anchoring plaques. On the basis of these observations, we postulate that COMP functions as an adapter protein in human skin, similar to its function in cartilage ECM, by organizing collagen I fibrils into a suprastructure, mainly in the vicinity of anchoring plaques that stabilize the cohesion between the upper dermis and the basement membrane zone.
Figures
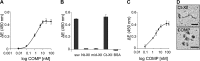



References
-
- Kadler K. E., Baldock C., Bella J., Boot-Handford R. P. (2007) Collagens at a glance. J. Cell Sci. 120, 1955–1958 - PubMed
-
- Myllyharju J., Kivirikko K. I. (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20, 33–43 - PubMed
-
- Sorrell J. M., Caplan A. I. (2004) Fibroblast heterogeneity. More than skin deep. J. Cell Sci. 117, 667–675 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

