Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients
- PMID: 22573349
- PMCID: PMC4015630
- DOI: 10.1158/1078-0432.CCR-12-0002
Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients
Abstract
Purpose: To investigate the angiogenic changes in primary tumor tissue of renal cell carcinoma (RCC) patients treated with VEGF-targeted therapy.
Experimental design: Phase II trials of VEGF pathway-targeted therapy given before cytoreductive surgery were carried out with metastatic RCC patients with the primary tumor in situ to investigate the necessity of nephrectomy. Primary tumor tissues were obtained and assessed for angiogenesis parameters. Results were compared with similar analyses on untreated tumors.
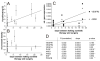
Results: Sunitinib or bevacizumab pretreatment resulted in a significant reduction of microvessel density in the primary tumor. Also, an increase in vascular pericyte coverage was found in sunitinib-pretreated tumors, consistent with efficient angiogenesis inhibition. Expression of several key regulators of angiogenesis was found to be suppressed in pretreated tissues, among which VEGFR-1 and VEGFR-2, angiopoietin-1 and angiopoietin-2 and platelet-derived growth factor-B. In addition, apoptosis in tumor and endothelial cells was induced. Interestingly, in sunitinib-pretreated tissues a dramatic increase of the number of proliferating endothelial cells was observed, which was not the case in bevacizumab-pretreated tumors. A positive correlation with the interval between halting the therapy and surgery was found, suggesting a compensatory angiogenic response caused by the discontinuation of sunitinib treatment.
Conclusion: This study describes, for the first time, the angiostatic response in human primary renal cancers at the tissue level upon treatment with VEGF-targeted therapy. Discontinuation of treatment with tyrosine kinase inhibitors leads to accelerated endothelial cell proliferation. The results of this study contribute important data to the ongoing discussion on the discontinuation of treatment with kinase inhibitors.
Figures




References
-
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–68. - PubMed
-
- Teicher BA, Holden SA, Ara G, Korbut T, Menon K. Comparison of several antiangiogenic regimens alone and with cytotoxic therapies in the Lewis lung carcinoma. Cancer Chemother Pharmacol. 1996;38:169–77. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

