Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn's disease: an open-label, multicenter, prospective trial
- PMID: 22573600
- PMCID: PMC3437245
- DOI: 10.1002/ibd.23012
Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn's disease: an open-label, multicenter, prospective trial
Abstract
Background: Extracorporeal photopheresis (ECP) involves ex vivo leukocyte treatment with methoxsalen and UVA light to generate a tolerogenic response. A previous trial demonstrated that ECP permits corticosteroid withdrawal in steroid-dependent Crohn's disease (CD) patients who were in clinical remission. We studied the effect of ECP on steroid withdrawal in steroid-dependent CD.
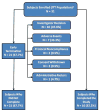
Methods: Patients with CD for ≥ 6 months, in remission at baseline while on steroids, but who had failed at ≥ 1 steroid withdrawal were included. Patients received two ECP treatments every 2 weeks for the 24-week steroid tapering period and underwent steroid-tapering. Patients completing steroid tapering could receive maintenance ECP (two treatments/week) every month for 24 weeks.
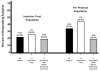
Results: Thirty-one patients (Crohn's Disease Activity Index [CDAI] score 91; Inflammatory Bowel Disease Questionnaire [IBDQ] 172.5) were enrolled (baseline corticosteroid dose, 20 mg/day); 65% were refractory to/intolerant of anti-tumor necrosis factor (TNF) agents or immunosuppressants. After 24 weeks of ECP, 7 of 31 (22.6%) patients discontinued steroids while maintaining a CDAI of <150. At week 24, the steroid dose for the remaining patients on corticosteroids was 10 mg (P < 0.003 vs. baseline) with a CDAI of 110 and an IBDQ of 179. Following maintenance treatment, three patients remained in steroid-free remission. The 10 patients in the study and receiving ECP at week 48 had a steroid dose of 3.5 mg with a CDAI of 40 and an IBDQ of 188.
Conclusions: ECP permitted discontinuation or reduction of steroids in a population of refractory steroid-dependent CD patients. ECP may be useful in permitting steroid withdrawal in selected steroid-dependent CD patients. Ideally, these results need to be confirmed in a "sham-controlled clinical trial.
Conflict of interest statement
This work was supported by a grant from Therakos, Inc., Raritan, NJ
Figures
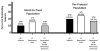
References
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;12;369(9573):1641–57. - PubMed
-
- Yang YX, Lichtenstein GR. Corticosteroids in Crohn’s disease. Am J Gastroenterol. 2002;97:803–23. - PubMed
-
- Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–60. - PubMed
-
- Reinisch W, Gasché C, Wyatt J, et al. Steroid dependency in Crohn’s disease. Lancet. 1995;1:345(8953):859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
