Microglia modulate hippocampal neural precursor activity in response to exercise and aging
- PMID: 22573666
- PMCID: PMC6621117
- DOI: 10.1523/JNEUROSCI.5925-11.2012
Microglia modulate hippocampal neural precursor activity in response to exercise and aging
Abstract
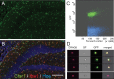
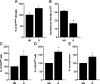
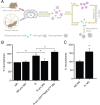
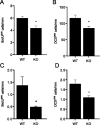
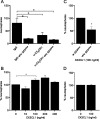
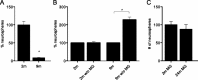
Exercise has been shown to positively augment adult hippocampal neurogenesis; however, the cellular and molecular pathways mediating this effect remain largely unknown. Previous studies have suggested that microglia may have the ability to differentially instruct neurogenesis in the adult brain. Here, we used transgenic Csf1r-GFP mice to investigate whether hippocampal microglia directly influence the activation of neural precursor cells. Our results revealed that an exercise-induced increase in neural precursor cell activity was mediated via endogenous microglia and abolished when these cells were selectively removed from hippocampal cultures. Conversely, microglia from the hippocampi of animals that had exercised were able to activate latent neural precursor cells when added to neurosphere preparations from sedentary mice. We also investigated the role of CX(3)CL1, a chemokine that is known to provide a more neuroprotective microglial phenotype. Intraparenchymal infusion of a blocking antibody against the CX(3)CL1 receptor, CX(3)CR1, but not control IgG, dramatically reduced the neurosphere formation frequency in mice that had exercised. While an increase in soluble CX(3)CL1 was observed following running, reduced levels of this chemokine were found in the aged brain. Lower levels of CX(3)CL1 with advancing age correlated with the natural decline in neural precursor cell activity, a state that could be partially alleviated through removal of microglia. These findings provide the first direct evidence that endogenous microglia can exert a dual and opposing influence on neural precursor cell activity within the hippocampus, and that signaling through the CX(3)CL1-CX(3)CR1 axis critically contributes toward this process.
Figures

References
-
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor β increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. - PubMed
-
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
