Photochemical inactivation analysis of temporal dynamics of postsynaptic native AMPA receptors in hippocampal slices
- PMID: 22573674
- PMCID: PMC6621109
- DOI: 10.1523/JNEUROSCI.0720-12.2012
Photochemical inactivation analysis of temporal dynamics of postsynaptic native AMPA receptors in hippocampal slices
Abstract
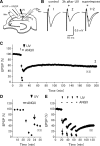
Postsynaptic expression of AMPA-type glutamate receptors (AMPAR) is more mobile than previously thought. Much evidence suggests that AMPAR are delivered from intracellular reserved pools to postsynaptic sites in a constitutive, as well as activity-dependent manner by exocytosis, lateral diffusion, or diffusional trapping. These notions were supported by optical monitoring of AMPAR subunits labeled with macromolecular tags such as GFP or Immunobeads, although it remains uncertain whether the mode and rate of synaptic delivery are similar to native "unlabeled" receptors. To reveal the real-time dynamics of native AMPAR in situ, photochemical inactivation of surface receptors using 6-azido-7-nitro-1,4-dihydroquinoxaline-2,3-dione (ANQX), a photoreactive AMPAR blocker, was adopted for acute hippocampal slices of mice. Because of the irreversible block due to cross-link formation between ANQX and surface AMPAR, recovery of EPSPs after photoinactivation reflects the time course of synaptic delivery of intracellular AMPAR. Brief UV illumination with fast application of ANQX resulted in persistent suppression of EPSPs for a prolonged period of up to 3 h, suggesting minimal synaptic delivery of AMPAR by exocytosis in the resting condition. Kinetic analysis of EPSP recovery clarified that the supply of postsynaptic AMPAR from the intracellular pool is dominated in the initial, but not in the later, phase of long-term potentiation (LTP). These results suggest that constitutive synaptic delivery is minimal in the resting condition at intact hippocampal synapses in a time scale of hours, while postsynaptic AMPAR are replaced with those in intracellular pools almost exclusively in an activity-dependent manner, typically shortly after LTP induction.
Figures






References
-
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. - PubMed
