Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation
- PMID: 22573687
- PMCID: PMC3412138
- DOI: 10.1523/JNEUROSCI.4876-11.2012
Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation
Abstract
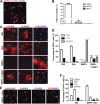
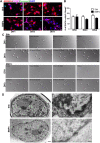
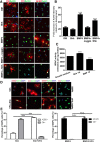
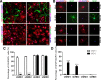
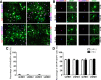
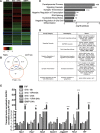
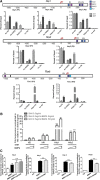
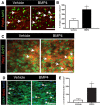
Differentiation of oligodendrocyte progenitor cells (OPCs) into mature oligodendrocytes is regulated by the interplay between extrinsic signals and intrinsic epigenetic determinants. In this study, we analyze the effect that the extracellular ligands sonic hedgehog (Shh) and bone morphogenetic protein 4 (BMP4), have on histone acetylation and gene expression in cultured OPCs. Shh treatment favored the progression toward oligodendrocytes by decreasing histone acetylation and inducing peripheral chromatin condensation. BMP4 treatment, in contrast, inhibited the progression toward oligodendrocytes and favored astrogliogenesis by favoring global histone acetylation and retaining euchromatin. Pharmacological treatment or silencing of histone deacetylase 1 (Hdac1) or histone deacetylase 2 (Hdac2) in OPCs did not affect BMP4-dependent astrogliogenesis, while it prevented Shh-induced oligodendrocyte differentiation and favored the expression of astrocytic genes. Transcriptional profiling of treated OPCs, revealed that BMP4-inhibition of oligodendrocyte differentiation was accompanied by increased levels of Wnt (Tbx3) and Notch-target genes (Jag1, Hes1, Hes5, Hey1, and Hey2), decreased recruitment of Hdac and increased histone acetylation at these loci. Similar upregulation of Notch-target genes and increased histone acetylation were observed in the corpus callosum of mice infused with BMP4 during cuprizone-induced demyelination. We conclude that Shh and Bmp4 differentially regulate histone acetylation and chromatin structure in OPCs and that BMP4 acts as a potent inducer of gene expression, including Notch and Wnt target genes, thereby enhancing the crosstalk among signaling pathways that are known to inhibit myelination and repair.
Figures
Similar articles
-
The Autotaxin-Lysophosphatidic Acid Axis Modulates Histone Acetylation and Gene Expression during Oligodendrocyte Differentiation.J Neurosci. 2015 Aug 12;35(32):11399-414. doi: 10.1523/JNEUROSCI.0345-15.2015. J Neurosci. 2015. PMID: 26269646 Free PMC article.
-
Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4.J Neurosci Res. 2004 Apr 1;76(1):9-19. doi: 10.1002/jnr.20047. J Neurosci Res. 2004. PMID: 15048926
-
Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury.Am J Physiol Renal Physiol. 2010 Jan;298(1):F133-41. doi: 10.1152/ajprenal.00400.2009. Epub 2009 Nov 11. Am J Physiol Renal Physiol. 2010. PMID: 19906951
-
HDAC1 and HDAC2 in mouse oocytes and preimplantation embryos: Specificity versus compensation.Cell Death Differ. 2016 Jul;23(7):1119-27. doi: 10.1038/cdd.2016.31. Epub 2016 Apr 15. Cell Death Differ. 2016. PMID: 27082454 Free PMC article. Review.
-
Histone deacetylase‑2: A potential regulator and therapeutic target in liver disease (Review).Int J Mol Med. 2021 Jul;48(1):131. doi: 10.3892/ijmm.2021.4964. Epub 2021 May 20. Int J Mol Med. 2021. PMID: 34013366 Free PMC article. Review.
Cited by
-
E2F1 coregulates cell cycle genes and chromatin components during the transition of oligodendrocyte progenitors from proliferation to differentiation.J Neurosci. 2014 Jan 22;34(4):1481-93. doi: 10.1523/JNEUROSCI.2840-13.2014. J Neurosci. 2014. PMID: 24453336 Free PMC article.
-
Mechanostimulation Promotes Nuclear and Epigenetic Changes in Oligodendrocytes.J Neurosci. 2016 Jan 20;36(3):806-13. doi: 10.1523/JNEUROSCI.2873-15.2016. J Neurosci. 2016. PMID: 26791211 Free PMC article.
-
The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model.PLoS One. 2015 Apr 30;10(4):e0124657. doi: 10.1371/journal.pone.0124657. eCollection 2015. PLoS One. 2015. PMID: 25927436 Free PMC article.
-
Developmental and Repairing Production of Myelin: The Role of Hedgehog Signaling.Front Cell Neurosci. 2018 Sep 6;12:305. doi: 10.3389/fncel.2018.00305. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30237763 Free PMC article.
-
Inhibiting Bone Morphogenetic Protein 4 Type I Receptor Signaling Promotes Remyelination by Potentiating Oligodendrocyte Differentiation.eNeuro. 2019 May 14;6(2):ENEURO.0399-18.2019. doi: 10.1523/ENEURO.0399-18.2019. Print 2019 Mar/Apr. eNeuro. 2019. PMID: 31028086 Free PMC article.
References
-
- Ara J, See J, Mamontov P, Hahn A, Bannerman P, Pleasure D, Grinspan JB. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:125–135. - PubMed
-
- Back SA, Craig A, Kayton RJ, Luo NL, Meshul CK, Allcock N, Fern R. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2007;27:334–347. - PubMed
-
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15:314–329. - PubMed
-
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
