Maternal HIV-1 disease progression 18-24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): The Kesho Bora randomized controlled trial
- PMID: 22573845
- PMCID: PMC3393708
- DOI: 10.1093/cid/cis461
Maternal HIV-1 disease progression 18-24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): The Kesho Bora randomized controlled trial
Abstract
Background: Antiretroviral (ARV) prophylaxis effectively reduces mother-to-child transmission of human immunodeficiency virus type 1 (HIV). However, it is unclear whether stopping ARVs after breastfeeding cessation affects maternal HIV disease progression. We assessed 18-24-month postpartum disease progression risk among women in a randomized trial assessing efficacy and safety of prophylactic maternal ARVs.
Methods: From 2005 to 2008, HIV-infected pregnant women with CD4(+) counts of 200-500/mm(3) were randomized to receive either triple ARV (zidovudine, lamivudine, and lopinavir/ritonavir during pregnancy and breastfeeding) or AZT/sdNVP (zidovudine until delivery with single-dose nevirapine without postpartum prophylaxis). Maternal disease progression was defined as the combined endpoint of death, World Health Organization clinical stage 4 disease, or CD4(+) counts of <200/mm(3).
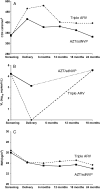
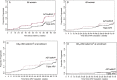
Results: Among 824 randomized women, 789 had at least 1 study visit after cessation of ARV prophylaxis. Following delivery, progression risk up to 24 months postpartum in the triple ARV arm was significantly lower than in the AZT/sdNVP arm (15.7% vs 28.3%; P = .001), but the risks of progression after cessation of ARV prophylaxis (rather than after delivery) were not different (15.0% vs 13.8% 18 months after ARV cessation). Among women with CD4(+) counts of 200-349/mm(3) at enrollment, 24.0% (95% confidence interval [CI], 15.7-35.5) progressed with triple ARV, and 23.0% (95% CI, 17.8-29.5) progressed with AZT/sdNVP, whereas few women in either arm (<5%) with initial CD4(+) counts of ≥350/mm(3) progressed.
Conclusions: Interrupting prolonged triple ARV prophylaxis had no effect on HIV progression following cessation (compared with AZT/sdNVP). However, women on triple ARV prophylaxis had lower progression risk during the time on triple ARV. Given the high rate of progression among women with CD4(+) cells of <350/mm(3), ARVs should not be discontinued in this group.
Clinical trials registration: ISRCTN71468410.
Figures
References
-
- The Kesho Bora Study Group. Safety and effectiveness of antiretroviral drugs during pregnancy, delivery and breastfeeding for prevention of mother-to-child transmission of HIV-1: the Kesho Bora Multicentre Collaborative Study rationale, design, and implementation challenges. Contemp Clin Trials. 2011;1:74–85. - PubMed
-
- The Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. - PubMed
-
- The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. - PubMed
-
- Danel C, Moh R, Minga A, et al. for the Trivacan ANRS 1269 trial group. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

