Progressive sheet-to-tubule transformation is a general mechanism for endoplasmic reticulum partitioning in dividing mammalian cells
- PMID: 22573885
- PMCID: PMC3386207
- DOI: 10.1091/mbc.E10-12-0950
Progressive sheet-to-tubule transformation is a general mechanism for endoplasmic reticulum partitioning in dividing mammalian cells
Abstract
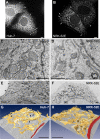
The endoplasmic reticulum (ER) is both structurally and functionally complex, consisting of a dynamic network of interconnected sheets and tubules. To achieve a more comprehensive view of ER organization in interphase and mitotic cells and to address a discrepancy in the field (i.e., whether ER sheets persist, or are transformed to tubules, during mitosis), we analyzed the ER in four different mammalian cell lines using live-cell imaging, high-resolution electron microscopy, and three dimensional electron microscopy. In interphase cells, we found great variation in network organization and sheet structures among different cell lines. In mitotic cells, we show that the ER undergoes both spatial reorganization and structural transformation of sheets toward more fenestrated and tubular forms. However, the extent of spatial reorganization and sheet-to-tubule transformation varies among cell lines. Fenestration and tubulation of the ER correlates with a reduced number of membrane-bound ribosomes.
Figures








References
-
- Black VH, Sanjay A, van Leyen K, Lauring B, Kreibich G. Cholesterol and steroid synthesizing smooth endoplasmic reticulum of adrenocortical cells contains high levels of proteins associated with the translocation channel. Endocrinology. 2005;146:4234–4249. - PubMed
-
- Bobinnec Y, Marcaillou C, Morin X, Debec A. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil Cytoskeleton. 2003;54:217–225. - PubMed
-
- Brown D. Fenestrae in the rough endoplasmic reticulum of Xenopus laevis hepatocytes. Anat Rec. 1978;191:103–110. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

