Obscurin and KCTD6 regulate cullin-dependent small ankyrin-1 (sAnk1.5) protein turnover
- PMID: 22573887
- PMCID: PMC3386213
- DOI: 10.1091/mbc.E12-01-0052
Obscurin and KCTD6 regulate cullin-dependent small ankyrin-1 (sAnk1.5) protein turnover
Abstract
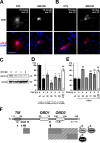
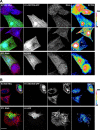
Protein turnover through cullin-3 is tightly regulated by posttranslational modifications, the COP9 signalosome, and BTB/POZ-domain proteins that link cullin-3 to specific substrates for ubiquitylation. In this paper, we report how potassium channel tetramerization domain containing 6 (KCTD6) represents a novel substrate adaptor for cullin-3, effectively regulating protein levels of the muscle small ankyrin-1 isoform 5 (sAnk1.5). Binding of sAnk1.5 to KCTD6, and its subsequent turnover is regulated through posttranslational modification by nedd8, ubiquitin, and acetylation of C-terminal lysine residues. The presence of the sAnk1.5 binding partner obscurin, and mutation of lysine residues increased sAnk1.5 protein levels, as did knockdown of KCTD6 in cardiomyocytes. Obscurin knockout muscle displayed reduced sAnk1.5 levels and mislocalization of the sAnk1.5/KCTD6 complex. Scaffolding functions of obscurin may therefore prevent activation of the cullin-mediated protein degradation machinery and ubiquitylation of sAnk1.5 through sequestration of sAnk1.5/KCTD6 at the sarcomeric M-band, away from the Z-disk-associated cullin-3. The interaction of KCTD6 with ankyrin-1 may have implications beyond muscle for hereditary spherocytosis, as KCTD6 is also present in erythrocytes, and erythrocyte ankyrin isoforms contain its mapped minimal binding site.
Figures






Similar articles
-
Electrostatic interactions mediate binding of obscurin to small ankyrin 1: biochemical and molecular modeling studies.J Mol Biol. 2011 Apr 29;408(2):321-34. doi: 10.1016/j.jmb.2011.01.053. Epub 2011 Feb 17. J Mol Biol. 2011. PMID: 21333652 Free PMC article.
-
Obscurin is a ligand for small ankyrin 1 in skeletal muscle.Mol Biol Cell. 2003 Mar;14(3):1138-48. doi: 10.1091/mbc.e02-07-0411. Mol Biol Cell. 2003. PMID: 12631729 Free PMC article.
-
Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum.J Cell Sci. 2009 Aug 1;122(Pt 15):2640-50. doi: 10.1242/jcs.046193. Epub 2009 Jul 7. J Cell Sci. 2009. PMID: 19584095 Free PMC article.
-
Building and remodelling Cullin-RING E3 ubiquitin ligases.EMBO Rep. 2013 Dec;14(12):1050-61. doi: 10.1038/embor.2013.173. Epub 2013 Nov 15. EMBO Rep. 2013. PMID: 24232186 Free PMC article. Review.
-
Protection of cullin-RING E3 ligases by CSN-UBP12.Trends Cell Biol. 2006 Jul;16(7):362-9. doi: 10.1016/j.tcb.2006.05.001. Epub 2006 Jun 9. Trends Cell Biol. 2006. PMID: 16762551 Review.
Cited by
-
Murine obscurin and Obsl1 have functionally redundant roles in sarcolemmal integrity, sarcoplasmic reticulum organization, and muscle metabolism.Commun Biol. 2019 May 9;2:178. doi: 10.1038/s42003-019-0405-7. eCollection 2019. Commun Biol. 2019. PMID: 31098411 Free PMC article.
-
Understanding the molecular basis of cardiomyopathy.Am J Physiol Heart Circ Physiol. 2022 Feb 1;322(2):H181-H233. doi: 10.1152/ajpheart.00562.2021. Epub 2021 Nov 19. Am J Physiol Heart Circ Physiol. 2022. PMID: 34797172 Free PMC article. Review.
-
Obscurin is required for ankyrinB-dependent dystrophin localization and sarcolemma integrity.J Cell Biol. 2013 Feb 18;200(4):523-36. doi: 10.1083/jcb.201205118. J Cell Biol. 2013. PMID: 23420875 Free PMC article.
-
Isolation and culture of neonatal mouse cardiomyocytes.J Vis Exp. 2013 Sep 6;(79):50154. doi: 10.3791/50154. J Vis Exp. 2013. PMID: 24056408 Free PMC article.
-
The M-band: The underestimated part of the sarcomere.Biochim Biophys Acta Mol Cell Res. 2020 Mar;1867(3):118440. doi: 10.1016/j.bbamcr.2019.02.003. Epub 2019 Feb 7. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 30738787 Free PMC article. Review.
References
-
- Alter P, Maisch B. Non-compaction cardiomyopathy in an adult with hereditary spherocytosis. Eur J Heart Fail. 2007;9:98–99. - PubMed
-
- Bang ML, et al. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89:1065–1072. - PubMed
-
- Bayon Y, et al. KCTD5, a putative substrate adaptor for cullin3 ubiquitin ligases. FEBS J. 2008;275:3900–3910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

