The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages
- PMID: 22574140
- PMCID: PMC3344844
- DOI: 10.1371/journal.pone.0036198
The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages
Abstract
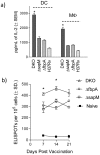
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is the leading cause of death due to bacterial infections in mankind, and BCG, an attenuated strain of Mycobacterium bovis, is an approved vaccine. BCG sequesters in immature phagosomes of antigen presenting cells (APCs), which do not fuse with lysosomes, leading to decreased antigen processing and reduced Th1 responses. However, an Mtb derived ΔfbpA attenuated mutant underwent limited phagosome maturation, enhanced immunogenicity and was as effective as BCG in protecting mice against TB. To facilitate phagosome maturation of ΔfbpA, we disrupted an additional gene sapM, which encodes for an acid phosphatase. Compared to the wild type Mtb, the ΔfbpAΔsapM (double knock out; DKO) strain was attenuated for growth in mouse macrophages and PMA activated human THP1 macrophages. Attenuation correlated with increased oxidants in macrophages in response to DKO infection and enhanced labeling of lysosomal markers (CD63 and rab7) on DKO phagosomes. An in vitro Antigen 85B peptide presentation assay was used to determine antigen presentation to T cells by APCs infected with DKO or other mycobacterial strains. This revealed that DKO infected APCs showed the strongest ability to present Ag85B to T cells (>2500 pgs/mL in 4 hrs) as compared to APCs infected with wild type Mtb or ΔfbpA or ΔsapM strain (<1000 pgs/mL in 4 hrs), indicating that DKO strain has enhanced immunogenicity than other strains. The ability of DKO to undergo lysosomal fusion and vacuolar acidification correlated with antigen presentation since bafilomycin, that inhibits acidification in APCs, reduced antigen presentation. Finally, the DKO vaccine elicited a better Th1 response in mice after subcutaneous vaccination than either ΔfbpA or ΔsapM. Since ΔfbpA has been used in mice as a candidate vaccine and the DKO (ΔfbpAΔsapM) mutant is more immunogenic than ΔfbpA, we propose the DKO is a potential anti-tuberculosis vaccine.
Conflict of interest statement
Figures

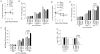

References
-
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. - PubMed
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
-
- Izzo A, Brandt L, Lasco T, Kipnis AP, Orme I. NIH pre-clinical screening program: overview and current status. Tuberculosis (Edinb) 2005;85:25–28. - PubMed
-
- Andersen P. TB vaccines: progress and problems. Trends Immunol. 2001;22:160–168. - PubMed
-
- Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

