Hypoxia-adaptation involves mitochondrial metabolic depression and decreased ROS leakage
- PMID: 22574227
- PMCID: PMC3344937
- DOI: 10.1371/journal.pone.0036801
Hypoxia-adaptation involves mitochondrial metabolic depression and decreased ROS leakage
Abstract
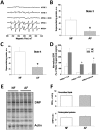
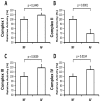
Through long-term laboratory selection, we have generated a Drosophila melanogaster population that tolerates severe, normally lethal, level of hypoxia. This strain lives perpetually under severe hypoxic conditions (4% O(2)). In order to shed light on the mechanisms involved in this adaptation, we studied the respiratory function of isolated mitochondria from the thorax of hypoxia-adapted flies (AF) using polarographic oxygen consumption while monitoring superoxide generation by electron paramagnetic resonance (EPR) techniques. AF mitochondria exhibited a significant 30% decrease in respiratory rate during state 3, while enhancing the resting respiratory rate during State 4-oligo by 220%. The activity of individual electron transport complexes I, II and III were 107%, 65%, and 120% in AF mitochondria as compared to those isolated from control flies. The sharp decrease in complex II activity and modest increase in complexes I and III resulted in >60% reduction in superoxide leakage from AF mitochondria during both NAD(+)-linked state 3 and State 4-oligo respirations. These results provide evidence that flies with mitochondria exhibiting decreased succinate dehydrogenase activity and reduced superoxide leakage give flies an advantage for survival in long-term hypoxia.
Conflict of interest statement
Figures

References
-
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. - PubMed
-
- Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007;42:165–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

